| Identification | More | [Name]
YTTRIUM SULFATE OCTAHYDRATE | [CAS]
7446-33-5 | [Synonyms]
YTTRIUM(III) SULFATE
YTTRIUM(III)SULFATE OCTAHYDRATE
YTTRIUM SULFATE
YTTRIUM SULFATE OCTAHYDRATE
YTTRIUM SULFATE OCTAHYDRATE, 99.9%
YTTRIUM(III) SULFATE OCTAHYDRATE 99.99&
YTTRIUM (III) SULFATE OCTAHYDRATE (99.9%-Y) (REO)
YTTRIUM (III) SULFATE, REACTON, 99.99% (REO)
Yttrium(III)sulfateoctahydrate,REacton,99.9%(REO)
yttrium(iii) sulfate octahydrate, reacton
YTTRIUMSULPHATEOCTAHYDRATE
Yttrium(III) sulfate octahydrate, REacton(R), 99.9% (REO)
Yttrium(III) sulfate hydrate, REacton(R), 99.99% (REO)
Yttrium(III) sulfate hydrate, REacton, 99.99% (REO) | [Molecular Formula]
H16O20S3Y2 | [MDL Number]
MFCD00149946 | [Molecular Weight]
610.12 | [MOL File]
7446-33-5.mol |
| Chemical Properties | Back Directory | [Appearance]
Small, reddish-white, monosymmetric
crystals. D 2.558, loses 8H2O at 120C, decomposes
700C. Soluble in concentrated sulfuric acid; sparingly
soluble in water; insoluble in alkalies. | [density ]
2.5 g/mL at 25 °C(lit.)
| [form ]
Crystalline | [color ]
White to pale yellow | [Specific Gravity]
2.558 | [Water Solubility ]
Water solubility decreases with increasing temperature. Soluble in water. | [Merck ]
14,10107 | [CAS DataBase Reference]
7446-33-5(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [TSCA ]
Yes |
| Questions And Answer | Back Directory | [Uses]
Yttrium Sulfate, also called Yttrium Sulphate, is applied in ceramics, glass, and electronics. Yttrium Sulfate is a moderately water and acid soluble Yttrium source for uses compatible with sulfates. Yttrium is used in the production of a large variety of synthetic garnets, and Yttria is used to make Yttrium-Iron-Garnets, which are very effective microwave filters.
|
| Hazard Information | Back Directory | [Description]
Yttrium(III) sulfate octahydrate, with the chemical formula Y2(SO4)3-8H2O, consists of infinite layers parallel to (10\overline 1), with [Y(H2O)4(SO4)3/2] being the only repeating unit. The layers are connected to each other using only moderately strong hydrogen bonds. The trivalent yttrium cation is coordinated by eight O-atoms from four sulfate groups and four water molecules in a distorted square antitetrahedron [YO8]. It is of the same structural type as the rare earth sulfate octahydrate[1].
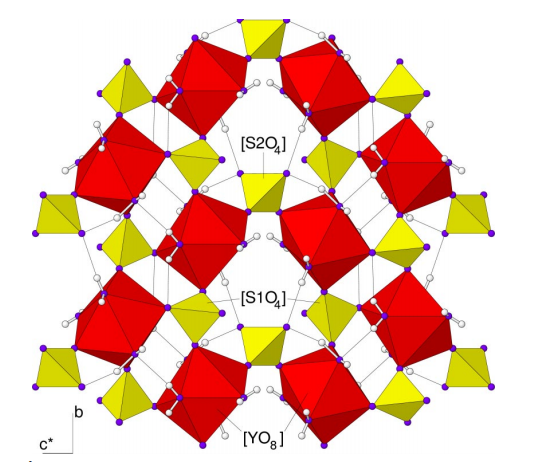
Figure 1. View perpendicular to the (10
1) layer of the title compound, with
[Y(H
2
O)
4/1
(SO
4
(1))
3/3
(SO
4
(2))
1/2
] as the repeat unit. Key: [SO
4
] tetra�hedra (yellow), [YO
8
] antiprisms (red), oxygen (blue) and hydrogen
(light grey) atoms. | [Chemical Properties]
Small, reddish-white, monosymmetric
crystals. D 2.558, loses 8H2O at 120C, decomposes
700C. Soluble in concentrated sulfuric acid; sparingly
soluble in water; insoluble in alkalies. | [Physical properties]
Red monoclinic crystals; density 2.59 g/cm3; loses all its water molecules at 120°C; decomposes at 700°C; sparingly soluble in water, less soluble in hot water; dissolves in concentrated sulfuric acid forming Y(HSO4)3; insoluble in alkalis; forms double salts with alkali sulfates. | [Preparation]
Yttrium sulfate is produced as an intermediate in recovering yttrium from monazite or xenotime (see Yttrium, Recovery). Rare earth sulfates are separated on a cation exchange resin bed. Yttrium fraction is purified by fractional crystallization. Alternatively, yttrium sulfate may be prepared by reacting yttrium oxide with sulfuric acid. | [References]
[1] PETER HELD; Ma-thias W. Yttrium(III) sulfate octahydrate[J]. Acta crystallographica. Section E, Structure reports online, 2004. DOI:10.1107/S1600536803012182. |
|
|