| Identification | Back Directory | [Name]
TAK-385 | [CAS]
737789-87-6 | [Synonyms]
TAK-385
CS-2851
RVT-601
Relugoli
Relugolix
Altropane
TKA-385 Relugolix
Relugolix TAK-385
TAK-385,Relugolix
10G,100G,500G,1KG
Relugolix Impurity 17
TAK-385;TAK 385;TAK385
1-[4-[1-[(2,6-difluorophenyl)methyl]-5-(dimethylaminomethyl)-3-(6-methoxy-3-pyridazinyl)-2,4-dioxo-6-thieno[4,5-e]pyrimidinyl]phenyl]-3-methoxyurea
1-[4-[1-[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxothieno[2,3-d]pyrimidin-6-yl]phenyl]-3-methoxyurea
N-[4-[1-[(2,6-Difluorophenyl)methyl]-5-[(dimethylamino)methyl]-1,2,3,4-tetrahydro-3-(6-methoxy-3-pyridazinyl)-2,4-dioxothieno[2,3-d]pyrimidin-6-yl]phenyl]-N'-methoxy urea
Urea, N-[4-[1-[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-1,2,3,4-tetrahydro-3-(6-methoxy-3-pyridazinyl)-2,4-dioxothieno[2,3-d]pyrimidin-6-yl]phenyl]-N'-methoxy-
1-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydro thieno[2,3-d]pyrimidin-6-yl)phenyl)-3-methoxy urea, Relugolix | [EINECS(EC#)]
237-099-7 | [Molecular Formula]
C29H27F2N7O5S | [MDL Number]
MFCD25976856 | [MOL File]
737789-87-6.mol | [Molecular Weight]
623.63 |
| Chemical Properties | Back Directory | [Melting point ]
228 °C (decomp)(Solv: ethyl acetate (141-78-6); tetrahydrofuran (109-99-9)) | [density ]
1.442±0.06 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
DMSO:20.0(Max Conc. mg/mL);32.1(Max Conc. mM)
Ethanol:1.0(Max Conc. mg/mL);1.6(Max Conc. mM) | [form ]
A crystalline solid | [pka]
13.17±0.70(Predicted) | [color ]
White to off-white | [InChIKey]
AOMXMOCNKJTRQP-UHFFFAOYSA-N | [SMILES]
N(C1=CC=C(C2SC3=C(C=2CN(C)C)C(=O)N(C2=NN=C(OC)C=C2)C(=O)N3CC2=C(F)C=CC=C2F)C=C1)C(NOC)=O |
| Hazard Information | Back Directory | [Description]
Relugolix (TAK-385) (737789-87-6);is a potent, orally active, nonpeptidic gonadotropin-releasing hormone (GnRH) antagonist. It possesses high affinity and potent antagonistic activity for human receptor (binding IC50=0.33 nM) and monkey receptor (IC50=0.32 nM) compared with TAK-013 (HY-100209). Relugolix is used for the study of sex-hormone-dependent diseases, such as including endometriosis, uterine fibroids and prostate cancer et al.
| [Uses]
Relugolix(737789-87-6) is a highly selective, oral, nonpeptide GnRH antagonist being investigated as a possible prostate cancer treatment
| [Mechanism of action]
The mechanism of action of relugolix(737789-87-6) is as a Gonadotropin Releasing Hormone Receptor Antagonist, and Cytochrome P450 3A Inducer, and Cytochrome P450 2B6 Inducer, and Breast Cancer Resistance Protein Inhibitor, and P-Glycoprotein Inhibitor. The physiologic effect of relugolix is by means of Decreased GnRH Secretion.
| [Pharmacokinetics]
Relugolix is a selective antagonist of the gonadotropin-releasing hormone receptor (GnRHR) (IC50 = 0.12 nM).A single oral administration of relugolix at a dose of 3 mg/kg has been found to suppress luteinizing hormone (LH) levels for more than 24 hours in castrated cynomolgus monkeys, indicating a long duration of action. The drug (80–160 mg/day) has been found to reduce testosterone levels to sustained castrate levels in men with once-daily administration.[8] Lower dosages (10–40 mg/day) are being studied in the treatment of endometriosis and uterine fibroids to achieve partial sex hormone suppression. The reasoning behind partial suppression for these conditions is to reduce the incidence and severity of menopausal symptoms such as hot flushes and to avoid bone mineral density changes caused by estrogen deficiency that can eventually lead to osteoporosis. | [Clinical Use]
Relugolix was first approved in Japan in 2019, under the brand name Relumina, for the symptomatic treatment of uterine fibroids, and more recently by the United States' FDA in 2020, under the brand name Orgovyx, for the treatment of advanced prostate cancer. Relugolix has also been studied in the symptomatic treatment of endometriosis. | [Side effects]
Common side effects of Relugolix(737789-87-6) include: hot flashes; flushing of the skin; sweating; weight gain or loss of ability; pain in the muscles, back, joints or bones; fatigue; diarrhoea; constipation; difficulty sleeping; depression; and breast enlargement.More serious side effects include: dizziness; fainting; rapid heartbeat; or chest pain; swelling of the face, lips, mouth or tongue; difficulty breathing or swallowing; rash; measles; redness of the skin; chest pain or tightness in the chest; or pain in the arms, back, neck or jaw; sudden numbness or weakness of the face, arms, or legs (especially on one side of the body); sudden blurred consciousness; difficulty speaking or understanding; sudden difficulty seeing with one or both eyes; or sudden difficulty walking, walking, or seeing with one or both eyes. or sudden difficulty walking, dizziness, loss of balance or coordination, etc.
| [Synthesis]
Relugolix is produced by the reaction of 6-(4-aminophenyl)-1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxypyridazin-3-yl)thieno[2,3-d]pyrimidine-2,4(1H,3H)-dione and phenyl N-methoxycarbamate.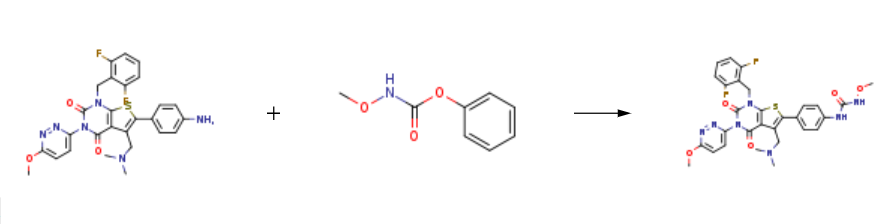 | [Enzyme inhibitor]
This phenyltropane derivative (FW = 429.27 g/mol; CAS 180468-34-2),
also known as O-587, IACFT, and 2β-carbomethoxy-3β- (4-fluorophenyl) -
N- ( (E) -3-iodoprop-2-enyl) tropane, is a dopamine reuptake inhibitor that
displays high affinity and specificity both in vitro and in vivo in laboratory
animals. The favorable binding properties of altropane, together with its
rapid entry into primate brain and highly localized distribution in
dopamine-rich brain regions, suggest it is a suitable iodinated probe for
monitoring the dopamine transporter in vitro and in vivo by SPECT or PET
imaging. Altropane also shows promise for treating attention deficit
hyperactivity disorder (ADHD). |
|
|