| Identification | More | [Name]
N-alpha-L-(Butoxycarbonyl)-2,3-diaminopropionic acid | [CAS]
73259-81-1 | [Synonyms]
3-AMINO-(TERT-BUTOXYCARBONYL)-L-ALANINE
BOC-ALPHA,BETA-DIAMINOPROPIONIC ACID
BOC-DAP-OH
BOC-DPR-OH
BOC-L-2,3-DIAMINOPROPIONIC ACID
BOC-L-ALPHA,BETA-DIAMINOPROPIONIC ACID
BOC-L-DAPA-OH
BOC-L-DAP-OH
L-ALANINE, 3-AMINO-N-[(1,1-DIMETHYLETHOXY)CARBONYL]-
N-ALPHA-BOC-BETA-AMINO-L-ALANINE
N-ALPHA-BOC-L-2,3-DIAMINOPROPIONIC ACID
NALPHA-BOC-L-BETA-AMINOALANINE
N-ALPHA-BOC-L-DIAMINOPROPIONIC ACID
N-ALPHA-BOC-(S)-2,3-DIAMINOPROPIONIC ACID
N-ALPHA-L-(BUTOXYCARBONYL)2,3-DIAMINOPROPIONIC ACID
N-ALPHA-T-BUTOXYCARBONYL-L-ALPHA,BETA-DIAMINOPROPIONIC ACID
N-ALPHA-TERT-BUTYLOXYCARBONYL-L-2,3-DIAMINOPROPIONIC ACID
(N-BOC-BETA-AMINO)-ALA-OH
N-T-BOC-BETA-AMINO-L-ALANINE
(S)-3-AMINO-2-(TERT-BUTOXYCARBONYLAMINO)PROPANOIC ACID | [EINECS(EC#)]
1533716-785-6 | [Molecular Formula]
C8H16N2O4 | [MDL Number]
MFCD00236843 | [Molecular Weight]
204.22 | [MOL File]
73259-81-1.mol |
| Chemical Properties | Back Directory | [Appearance]
White powder | [Melting point ]
210 °C (dec.)
| [alpha ]
-5.5 º (c=1, acetic acid) | [Boiling point ]
364.4±37.0 °C(Predicted) | [density ]
1.189±0.06 g/cm3(Predicted) | [storage temp. ]
Store at RT. | [solubility ]
DMSO (Slightly, Heated, Sonicated), Water (Sparingly, Heated, Sonicated) | [form ]
Solid | [pka]
2.88±0.16(Predicted) | [color ]
White to Off-White | [optical activity]
[α]20/D +5.5±1°, c = 1% in methanol: water (1:1) | [BRN ]
4182136 | [InChI]
InChI=1S/C8H16N2O4/c1-8(2,3)14-7(13)10-5(4-9)6(11)12/h5H,4,9H2,1-3H3,(H,10,13)(H,11,12)/t5-/m0/s1 | [InChIKey]
KRJLRVZLNABMAT-YFKPBYRVSA-N | [SMILES]
C(O)(=O)[C@H](CN)NC(OC(C)(C)C)=O | [CAS DataBase Reference]
73259-81-1(CAS DataBase Reference) |
| Safety Data | Back Directory | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [F ]
10 | [HazardClass ]
IRRITANT | [HS Code ]
29241990 |
| Hazard Information | Back Directory | [Chemical Properties]
White powder | [Uses]
Reactant for:
- Protein assembly directed by synthetic molecular recognition motifs
- Solid phase synthesis of gramicidin S cyclic analogs with antibiotic and hemolytic activities
- Synthesis of HCV protease inhibitor modified analogs
- Solid phase synthesis of peptidic V1a receptor agonists
- Directed peptide assembly at lipid-water interface
| [Definition]
Boc-Dap-OH is an amino acid molecule that is a zwitterion. Therefore, the addition of a base is required to prepare a nucleophilic amino acid. It is commonly acts as a nucleophile to attack the acyl carbon.
| [Preparation]
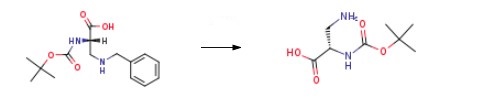
The raw material was dissolved inabsolute ethanol (5 mL), and the reaction flask was immersed in a water bath at 25 C. To the alcoholicsolution, 10% palladium-carbon (22.9 mg) and 1,4-cyclohexadiene (10 equivalents with respect to4; 0.47 mL, 5.0 mmol) were added, and the mixture was allowed to react under magnetic stirringovernight. After filtration over a short pad of Celite 545, the solvent was evaporated under reducedpressure conditions, and the solid residue was partitioned in a 1:1 (v/v) H2O/EtOAc mixture (10 mL).The aqueous phase was separated, back extracted with EtOAc (3 10 mL), concentrated under reducedpressure conditions, and lyophilized.Finally, the target product Boc-Dap-OH is obtained.
| [reaction suitability]
reaction type: Boc solid-phase peptide synthesis |
|
|