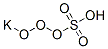| Identification | More | [Name]
Potassium peroxymonosulfate | [CAS]
70693-62-8 | [Synonyms]
Potassiumhydrogenperoxymonosulfate
potassiumperoxymonosulfatesulfate(k5h3(so3(o2))2(so4)2)
POTASSIUM CAROATE
PotassiumMonopersulphate,ActiveComponent42%Min
Potassium Monopersulphate, Active Component 42%Min, Cas
Potassium peroxymonosulfate sulfate (K5HSO3(O2)SO3(O2)(HSO4)2)
CAROAT (POTASSIUM MONOPERSULFATE)
Pentakalium-bis(peroxymonosulfat)-bis(sulfat)
Potassium Monopersulfate Sulfate
Pentapotassium bis(peroxymonosulphate) bis(sulphate)
Potassium peroxymonosulfate
Potassium peroxymonosulfate sulfate | [EINECS(EC#)]
274-778-7 | [Molecular Formula]
HKO6S | [MDL Number]
MFCD00040551 | [Molecular Weight]
168.17 | [MOL File]
70693-62-8.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystalline powder | [density ]
1.15 | [vapor pressure ]
<0.0000017 hPa | [storage temp. ]
Store at <= 20°C. | [solubility ]
250-300g/l soluble | [form ]
solid | [color ]
white | [Specific Gravity]
1.12-1.20 | [Odor]
Odorless | [PH]
2-3 (10g/l, H2O, 20℃) | [Stability:]
Stable. Oxidizer. Incompatible with combustible materials, bases. | [Water Solubility ]
Soluble in water (100 mg/ml). | [Sensitive ]
Hygroscopic | [Exposure limits]
ACGIH: TWA 0.1 mg/m3 | [InChIKey]
HVAHYVDBVDILBL-UHFFFAOYSA-M | [LogP]
-3.9 at 25℃ | [CAS DataBase Reference]
70693-62-8(CAS DataBase Reference) | [EPA Substance Registry System]
70693-62-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
O,C | [Risk Statements ]
R8:Contact with combustible material may cause fire.
R22:Harmful if swallowed.
R34:Causes burns.
R42/43:May cause sensitization by inhalation and skin contact . | [Safety Statements ]
S22:Do not breathe dust .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3260 8/PG 2
| [WGK Germany ]
1
| [TSCA ]
Yes | [HS Code ]
2833 40 00 | [HazardClass ]
5.1 | [PackingGroup ]
III | [Toxicity]
LD50 orally in Rabbit: > 2000 mg/kg |
| Hazard Information | Back Directory | [Chemical Properties]
white crystalline powder | [Uses]
PCB metal surface treatment chemical and water treatment etc. | [General Description]
OXONE?, monopersulfate compound is a potassium triple salt mainly used as a stable, easy to handle and nontoxic oxidant. | [Flammability and Explosibility]
Nonflammable | [reaction suitability]
reagent type: oxidant | [Purification Methods]
This is a stable form of Caro's acid and should contain >4.7% of active oxygen. It can be used in EtOH/H2O and EtOH/AcOH/H2O solutions. If active oxygen is too low. it is best to prepare it afresh from 1mole of KHSO5, 0.5mole of KHSO4 and 0.5mole of K2SO4. [Kennedy & Stock J Org Chem 25 1901 1960, Stephenson US Patent 2,802,722 1957.] A rapid preparation of Caro's acid is made by stirring finely powdered potassium persulfate (M 270.3) into ice-cold conc H2SO4 (7mL) and when homogeneous add ice (40-50g). It is stable for several days if kept cold. Keep away from organic matter as it is a STRONG OXIDANT. A detailed preparation of Caro's acid (hypersulfuric acid, H2SO5) in crystalline form m ~45o from H2O2 and chlorosulfonic acid was described by Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 388 1963. | [Toxics Screening Level]
The initial threshold screening level (ITSL) for acute exposure to pentapotassium bis(peroxymonosulphate) bis(sulphate) (KMPS) is 35 μg/m3 (8-hour averaging time) based on the Michigan Department of Environmental Quality (MDEQ), Air Quality Division (AQD) Rule 336.1233. |
| Questions And Answer | Back Directory | [Reactions]
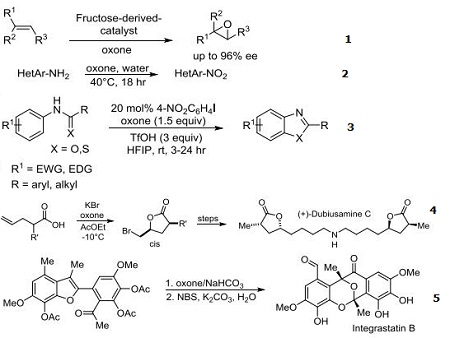
- Reagent for the catalytic asymmetric Shi epoxidation
- Reagent for the synthesis of nitro heteroaromatics in water
- Reagent for the syntheses of benzoxazoles and benzothiazoles using aryl iodides via C-H functionalization and C-O/S bond formation
- Reagent used for bromolactonization in the asymmetric total synthesis of (+)-Dubiusamine C
- Reagent for the benzofuran oxidative dearomatization cascade in the total synthesis of Integrastatin B
|
|
|