| Identification | More | [Name]
2,4,6-TRIPROPYL-1,3,5,2,4,6-TRIOXATRIPHOSPHORINANE-2,4,6-TRIOXIDE | [CAS]
68957-94-8 | [Synonyms]
1-PROPANEPHOSPHONIC ACID ANHYDRIDE CYCLIC TRIMER
1-PROPANEPHOSPHONIC ACID CYCLIC ANHYDRIDE
1-PROPANEPHOSPHONIC ANHYDRIDE
1-PROPYLPHOSPHONIC ACID CYCLIC ANHYDRIDE
1-PROPYLPHOSPHONIC CYCLIC ANHYDRIDE
2,4,6-TRIPROPYL-1,3,5,2,4,6-TRIOXATRIPHOSPHORINANE-2,4,6-TRIOXIDE
N-PROPYLPHOSPHONIC ACID ANHYDRIDE, CYCLIC TRIMER
PPAA
PROPANE PHOSPHONIC ACID ANHYDRIDE
PROPANEPHOSPHONIC ACID CYCLIC ANHYDRIDE
PROPYLPHOSPHONIC ACID ANHYDRIDE CYCLIC TRIMER
PROPYLPHOSPHONIC ANHYDRIDE
T3P
TRIS-N-PROPAN-PHOSPHONIC ACID ANHYDRIDE
1,3,5,2,4,6-trioxatriphosphorinane,2,4,6-tripropyl-,2,4,6-trioxide
propanephosphonicacidanhydride(t3p)
propylphosphonicanhydridesolution
1-Propanephosphonic acid cyclic anhydride~(rgT3P~2,4,6-Tripropyl-2,4,6-trioxo-1,3,5,2,4,6-trioxatriphosphorinane
2,4,6-tripropyl-1,3,5-trioxa-2,4,6-triphosphorinane-2,4,6-trioxide
Propanephosphonic acid cyclic anhydride (T3P) | [EINECS(EC#)]
422-210-5 | [Molecular Formula]
C9H21O6P3 | [MDL Number]
MFCD00006583 | [Molecular Weight]
318.18 | [MOL File]
68957-94-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Clear yellow solution | [Boiling point ]
65 °C
| [density ]
1.069 g/mL at 25 °C
| [vapor pressure ]
0Pa at 25℃ | [refractive index ]
n20/D 1.418
| [Fp ]
25 °F
| [storage temp. ]
Flammables area | [solubility ]
Miscible with dioxane, terahydrofuran, dimethyl formamide, polar and aprotic organic solvents. | [form ]
Solution | [color ]
Clear yellow to brownish | [Water Solubility ]
9.6g/L at 25℃ | [Sensitive ]
Moisture Sensitive | [BRN ]
5079255 | [Exposure limits]
ACGIH: TWA 20 ppm (Skin)
OSHA: TWA 40 ppm(70 mg/m3)
NIOSH: IDLH 137 ppm(25 mg/m3); TWA 20 ppm(34 mg/m3) | [Stability:]
Moisture Sensitive | [LogP]
0 at 25℃ | [CAS DataBase Reference]
68957-94-8(CAS DataBase Reference) | [Storage Precautions]
Moisture sensitive;Heat sensitive |
| Safety Data | Back Directory | [Hazard Codes ]
T,C,F | [Risk Statements ]
R61:May cause harm to the unborn child.
R20/21:Harmful by inhalation and in contact with skin .
R34:Causes burns.
R67:Vapors may cause drowsiness and dizziness.
R66:Repeated exposure may cause skin dryness or cracking.
R11:Highly Flammable. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S33:Take precautionary measures against static discharges .
S16:Keep away from sources of ignition-No smoking . | [RIDADR ]
UN 2924 3/PG 3
| [WGK Germany ]
1
| [F ]
21 | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
29319019 |
| Hazard Information | Back Directory | [Chemical Properties]
Clear light yellow solution | [Uses]
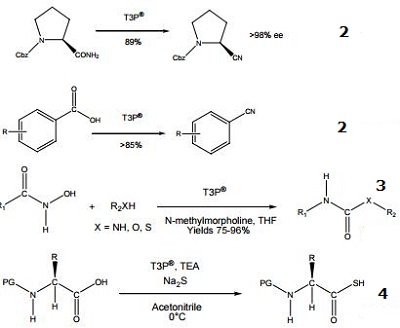
It is employed as an efficient promoter for the Lossen rearrangement to synthesis urea and carbamate derivatives. | [General Description]
Propylphosphonic anhydride (T3P) is a reactive n-propyl phosphonic acid cyclic anhydride. It is a mild and low toxic coupling agent used in peptide synthesis. T3P also acts as a promoter and water scavenger in the Friedl?nder annulation reaction. It participates in the conversion of carboxylic acids and amides into nitriles, formation of Weinreb amides, ester synthesis, dehydrations, oxidation of alcohols, isonitrile synthesis, synthesis of alkenes from alcohols and C-C coupling reactions. T3P delivers outstanding advantages over traditional reagents, such as broad functional group tolerance, low epimerization, and water-soluble by-products and hence gives high purity and yield of the product. | [Flammability and Explosibility]
Notclassified |
| Questions And Answer | Back Directory | [Reaction]
- TP3 is an exceptional reagent for amide/peptide bond formation. The product is very easy to use and combines excellent reaction selectivity, low epimerization, high yields and high product purities.
- Conversions of acids and amides to nitriles under mild conditions.
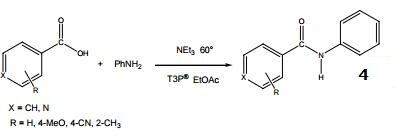
- Synthesis of urea and carbamate derivatives.
- Formation of thioacids from N-protected amino acids and peptides.
|
|