| Identification | More | [Name]
Terconazole | [CAS]
67915-31-5 | [Synonyms]
1-[4-[[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-propan-2-yl-piperazine
TERCONAZOLE
Terconagole
TERCONAZOLE EPT(CRM STANDARD)
TERCONAZOLE USP(CRM STANDARD)
Triaconazole
1-[4-[[(2R,4S)-2-(2,4-Dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-propan-2-yl-piperazine
Fungistat
Piperazine, 1-[4-[[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-(1-methylethyl)-, cis | [EINECS(EC#)]
267-751-6 | [Molecular Formula]
C26H31Cl2N5O3 | [MDL Number]
MFCD05662369 | [Molecular Weight]
532.46 | [MOL File]
67915-31-5.mol |
| Chemical Properties | Back Directory | [Appearance]
White or almost white powder. | [Melting point ]
126.3°C | [Boiling point ]
681.8±65.0 °C(Predicted) | [density ]
1.35±0.1 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
DMSO: soluble1mg/mL | [form ]
powder | [pka]
8.33±0.10(Predicted) | [color ]
white | [CAS DataBase Reference]
67915-31-5(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
Terconazole is an antifungal agent somewhat more potent than clotrimazole and
useful in the topical treatment of vaginal dermatophytosis and candidiasis. | [Chemical Properties]
White or almost white powder. | [Originator]
Janssen (Belgium) | [Uses]
Terazol (Ortho-McNeil). | [Definition]
ChEBI: (2R,4S)-terconazole is a 1-(4-{[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)-4-isopropylpiperazine in which positions 2 and 4 of the 1,3-dioxolane moiety have R and S configuration, respectively. It is an enantiomer of a (2S,4R)-terconazole. | [Indications]
Terconazole (Terazol) is a fungicidal triazole topical preparation effective
against many Candida strains. It is used as either a 3-day or a 7-day course
(Terazol 7—0.4% cream for 7 days or Terazol 3—0.8% cream for 3 days). | [Manufacturing Process]
A mixture of 1.6 parts of 1H-1,2,4-triazole, 54 parts of N.Ndimethylformamide
and 45 parts of benzene is stirred and refluxed for 2 h.
After cooling, 0.78 parts of sodium hydride dispersion 78% are added and the
whole is stirred for 30 min at room temperature. Then there are added 8.9
parts of cis-2-(bromomethyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-ylmethyl
benzoate and stirring is continued overnight at 150°C. The reaction mixture is
cooled and poured onto water. The product is extracted three times with
benzene. The combined extracts are washed twice with water, dried, filtered
and evaporated, yielding 8.5 parts of cis-[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-
triazol- 1-ylmethyl)-1,3-dioxolan-4-ylmethyl]benzoate as a residue.
A mixture of 289 parts of cis-[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-
ylmethyl)-1,3-dioxolan-4-ylmethyl]benzoate, 200 parts of sodium hydroxide
solution 50%, 1500 parts of 1,4-dioxane and 300 parts of water is stirred and
refiuxed for 2 h. The reaction mixture is cooled and poured onto water. The
product is extracted with dichloromethane. The extract is washed with water,
dried, filtered and evaporated. The residue is purified by columnchromatography
over silica gel using a mixture of trichloromethane and
methanol (95:5 by volume) as eluent. The first fraction is collected and the
eluent is evaporated, yielding 89 parts cis-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-
triazol-1-ylmethyl)-1,3-dioxolane-4-methanol; melting point 138.2°C.
A mixture of 30.6 parts of cis-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-
ylmethyl)-l,3-dioxolane-4-methanol and 75 parts of pyridine is stirred at room
temperature and there are added dropwise 17.2 parts of methanesulfonyl
chloride. Upon completion, stirring is continued overnight at room
temperature. The reaction mixture is poured onto ice-water and the product is
extracted twice with dichloromethane. The combined extracts are washed twice with a diluted hydrochloric acid solution and twice with water, dried,
filtered and evaporated. The residue is purified by column-chromatography
over silica gel using a mixture of trichloromethane and methanol (95:5 by
volume) as eluent. The first fraction is collected and the eluent is evaporated,
yielding 21 parts of cis-[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-
ylmethyl)-1,3-dioxolan-4-ylmethyl]methanesulfonate; melting point 98°C.
A mixture of 1-(4-hydroxyphenyl)-4-(1-methylethyl)piperazine, cis-[2-(2,4-
dichloro-phenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-
ylmethyl]methanesulfonate, potassium carbonate and N,N-dimethylformamide
is stirred and heated overnight at 120°C. The reaction mixture is cooled and
poured onto water. The product is extracted twice with dichloromethane. The
combined extracts are washed twice with a potassium carbonate solution,
dried, filtered and evaporated. The residue is taken up in methanol and a
sodium methanolate solution 30% are added. The whole is stirred and
refluxed for 1 h. The mixture is poured onto water and the layers are
separated. The organic phase is dried, filtered and evaporated. The cis-1-(4-
((2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-
yl)methoxy)phenyl)-4-(1-methylethyl)piperazine was obtained, melting point
116.3°C. | [Brand name]
Gyno-terazol;Terazol 3;Tercospor;FUNGISTAT. | [Therapeutic Function]
Antifungal | [World Health Organization (WHO)]
Terconazole, an antifungal agent, was introduced into medicine in
1980. It is indicated for the treatment of vaginal candidiasis. It is not yet clear
whether the adverse effects associated with high dose formulations are due to
terconazole itself, to an excipient in the preparation or to fungal constituent. | [Mechanism of action]
Terconazole is effective for fungistatic action for many strains of Candida and dermato�phytes. The exact mechanism of its action is unknown, although it inhibits the action of
the enzyme lanosterol 1-demethylase of cytochrome P-450 of sensitive fungi (similar to
other azols described above), causing a reduction in the amount of ergosterin, which is
necessary for the organisms to construct membranes and to retain the appropriate perme�ability. It is only used externally for treating vulvovaginal candidoses. Synonyms of this
drug are terazol, tercospor, and others. | [Clinical Use]
cis-1-[4-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy]phenyl]-4-(1methylethyl)piperazine (Terazol), or terconazole, is a triazolederivative that is used exclusively for the control ofvulvovaginal moniliasis caused by C. albicans and otherCandida species. It is available in creams containing 0.4%and 0.8% of the free base intended for 7-day and 3-day treatmentperiods, respectively. Suppositories containing 80 mgof the free base are also available. | [Synthesis]
Terconazole, 1-[4-[[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-yl-methyl)- 1,3-dioxolan-4-yl]methoxy]phenyl]-4-(1-methylethyl)-piperazine (35.2.19), is chemically very similar to ketoconazole, the only difference being that instead of an imidazole ring it contains a triazole ring and the piperazine ring, instead of an acetyl group is substituted by an isopropyl group. It is synthesized from 2,4-dichloroacetophenone, which is reacted with glycerol in the presence of p-toluenesulfonic acid to make a ketal, 2-(2,4-dichlorophenyl)- 2-methyl-4-hydroxymethyl-1,3-dioxolane (35.2.13). Brominating this with molecular bromine at the methyl group and then acylating the free hydroxyl group with benzoyl chlo�ride gives 2-(2,4-dichlorophenyl)-2-bromomethyl-4-benzoyloxymethyl-1,3-dioxolane (35.2.14). Reacting this with 1,2,4-triazole in the presence of sodium, followed by the hydrolysis of the protecting benzoyl group with sodium hydroxide gives 2-(2,4- dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-4-hydroxymethyl-1,3-dioxolane (35.2.15). Treating this with methanesulfonyl chloride gives the corresponding mesylate 35.2.16.
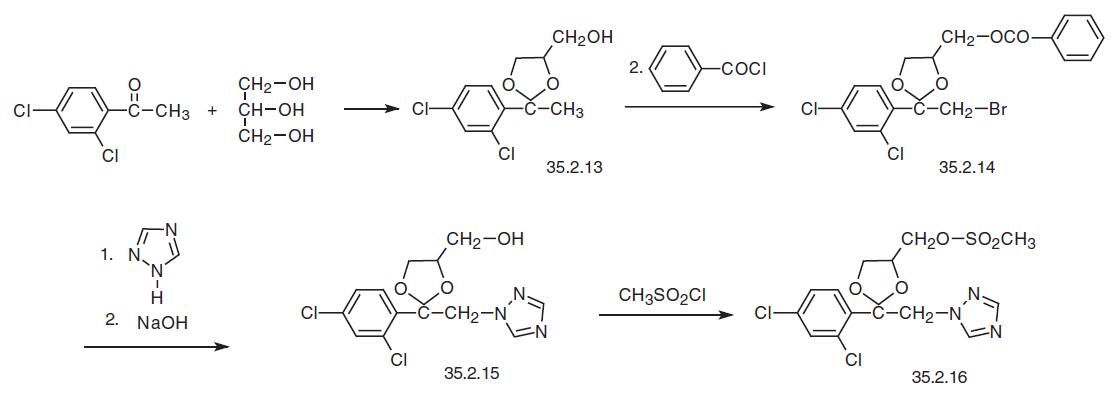
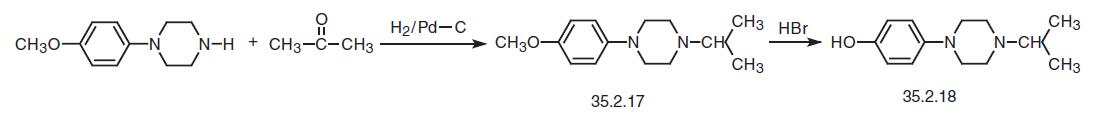
The way of making the second necessary fragment, 1-isopropyl-4-(4-hydroxyphenyl)- piperazine (35.2.18) is started from 4-(4-methoxyphenylpiperazine). Reducing this with hydrogen in the presence of acetone and using a palladium on carbon catalyst gives 1- isopropyl-4-(4-methoxyphenyl)piperazine (35.2.17). Treating of the resulting product with concentrated hydrobromic acid removes the protection from the phenol hydroxyl, making 1-isopropyl-4-(4-hydroxyphenyl)piperazine (35.2.18).
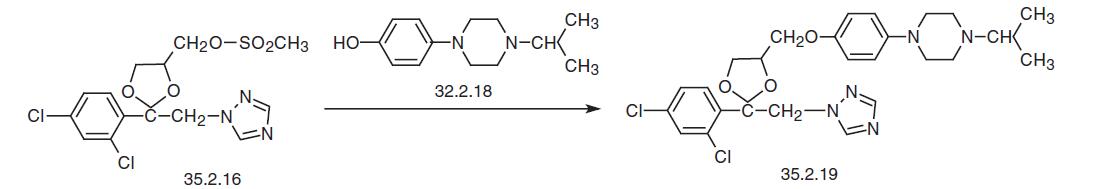
Finally, reacting the mesylate (35.2.16) with the resulting 1-isopropyl-4-(4-hydrox�yphenyl)piperazine (35.2.18) gives the desired terconazole.
|
|
|