| Identification | More | [Name]
1,8-Diazabicyclo[5.4.0]undec-7-ene | [CAS]
6674-22-2 | [Synonyms]
1,5-DIAZABICYCLO(5,4,0)UNDEC-5-ENE
1,8-DIAZABICYCLO[5,4,0]-7-UNDECENE
1,8-DIAZABICYCLO[5.4.0]UNDEC-7-ENE
1,8-DIAZABICYCLO[5.4.0]UNDEC-7-ENE (1,5-5)
1,8-DIAZABICYCLO(5,4,0)UNDECENE-7
1,8-DIAZEBICYCLO[5.4.0]UNDEC-7-ENE
2,3,4,6,7,8,9,10-OCTAHYDROPYRIMIDO[1,2-A]AZEPINE
2,3,4,6,7,8,9,10-OCTAHYDROPYRIMIDOL[1,2-A]AZEPINE
2,3,4,6,7,8,9,10-OCTA-HYDRO-PYRIMIDOL[1,2-ALPHA]AZEPINE
DBU
LUPRAGEN(R) N 700
OCTAHYDROPYRIMIDO(1,2:A)AZEPINE
POLYCAT(R) 18
POLYCAT(R) 48
1,5-Diaza(5,4,0)undec-5-ene
1,5-Diazabicyclo[5.4.0]-5-undecene
2,3,4,6,7,8,9,10-Octahydropyrimido(1,2-alpha)azepine
2-a]azepine,2,3,4,6,7,8,9,10-octahydro-pyrimido[
2-a]azepine,2,3,4,6,7,8,9,10-octahydro-Pyrimido[1
Diazabicycloundecene | [EINECS(EC#)]
229-713-7 | [Molecular Formula]
C9H16N2 | [MDL Number]
MFCD00006930 | [Molecular Weight]
152.24 | [MOL File]
6674-22-2.mol |
| Chemical Properties | Back Directory | [Description]
1,8-Diazabicyclo[5.4.0]undec-7-ene is a bicyclic amidine base. It is non-nucleophilic, sterically hindered, tertiary amine base in organic chemistry. It is reported to be superior to amine catalyst in Baylis-Hillman reaction.It promotes the methylation reaction of phenols, indoles and benzimidazoles with dimethyl carbonate under mild conditions. | [Appearance]
Colorless to yellow liquid | [Melting point ]
-70 °C
| [Boiling point ]
80-83 °C0.6 mm Hg(lit.)
| [density ]
1.019 g/mL at 20 °C(lit.)
| [vapor pressure ]
5.3 mm Hg ( 37.7 °C)
| [refractive index ]
n20/D 1.523
| [Fp ]
>230 °F
| [storage temp. ]
Store at R.T. | [solubility ]
soluble | [form ]
Liquid | [pka]
13.28±0.20(Predicted) | [color ]
Clear colorless to light yellow | [Odor]
Unpleasant | [PH]
12.8 (10g/l, H2O, 20℃) | [PH Range]
12.8 at 10 g/l at 20 °C | [Stability:]
Stable. Incompatible with strong oxidizing agents, acids, acid chlorides, acid anhydrides. | [explosive limit]
1.1-6.5%(V) | [Water Solubility ]
soluble | [Sensitive ]
Air Sensitive | [Detection Methods]
GC,NMR | [BRN ]
508906 | [InChIKey]
GQHTUMJGOHRCHB-UHFFFAOYSA-N | [Uses]
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) may be used:
- as catalyst for carboxylic acid esterification with dimethyl carbonate
- in the synthesis of duocarmycin and CC-1065 analogs
- as catalyst in aza-Michael addition and Knovenegal condensation reaction
- as base for dehalogenation of halogenated Diels-Alder adducts and the resulting activated 2,4-dienones were subjected to regio- and stereo-directed Michael additions, using Yamamoto′s reagent (CH3Cu · BF3)
- in a new synthesis of the ABCD ring system of Camptothecin
- 1,8-Diazabicyclo[5.4.0]undec-7-ene may be used as an catalyst for the dissolution and activation of cellulose by a reversible reaction of its hydroxyl groups with carbon dioxide. This dissolved cellulose system can be derivatized to form cellulose mixed esters.
- Used in a new synthesis of the ABCD ring system of Camptothecin.
| [CAS DataBase Reference]
6674-22-2(CAS DataBase Reference) | [NIST Chemistry Reference]
1,8-diazabicyclo [5.4.0]undec-7-ene(6674-22-2) | [Storage Precautions]
Air sensitive | [EPA Substance Registry System]
6674-22-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R22:Harmful if swallowed.
R34:Causes burns.
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R35:Causes severe burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S27:Take off immediately all contaminated clothing . | [RIDADR ]
UN 3267 8/PG 2
| [WGK Germany ]
2
| [F ]
34 | [Autoignition Temperature]
260 °C | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
29339930 | [Toxicity]
LD50 orally in Rabbit: 215 - 681 mg/kg |
| Hazard Information | Back Directory | [Chemical Properties]
Colorless to yellow liquid | [Occurrence]
Although all commercially available DBU is produced synthetically, it may also be isolated from the sea sponge Niphates digitalis.The biosynthesis of DBU has been proposed to begin with 1,6-hexanedial and 1,3-diaminopropane. | [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information. | [Flammability and Explosibility]
Nonflammable | [Synthesis]
The synthesis of DBU is as follows:As a base, sodium hydroxide was used at a concentration of 25% by weight and 2.3 times molar equivalent. A stirrer was placed in a 100 mL screw tube, and 11.5 g (50 mmol) of DBU hydrochloride, 8.5 g of toluene and 18.5 g (116 mmol) of 25% sodium hydroxide aqueous solution were charged at room temperature. It was placed on a magnetic stirrer and mixed for 1 hour with stirring. The mixture was separated with a 100 mL separatory funnel to obtain 18.8 g of the upper layer containing DBU and 18.4 g of the lower layer of the aqueous layer. The upper layer portion was analyzed by gas chromatography and contained 31.9% by weight of DBU, and the yield was 6.0 g (39.4 mmol) in a yield of 78.8%.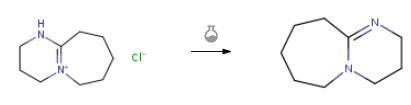
| [Purification Methods]
Fractionally distil DBU under vacuum. Also purify it by chromatography on Kieselgel and eluting with CHCl3/EtOH/25% aqueous NH3 (15:5:2) and checking by IR and MS. [Oediger et al. Chem Ber 99 2012 1962, Angew Chem, Int Ed Engl 6 76 1967, Guggisberg et al. Helv Chim Acta 61 1057 1978, Beilstein 23/5 V 271.] | [Toxics Screening Level]
The Initial Threshold Screening Level (ITSL) for 1,8-diazabicyclo(5.4.0)undec-7-ene (DBU) is 25 μg/m3 with an annual averaging time. |
|
|