| Identification | Back Directory | [Name]
(1,5-CYCLOOCTADIENE)(PYRIDINE)(TRICYCLOHEXYLPHOSPHINE)IRIDIUM(I) HEXAFLUOROPHOSPHATE | [CAS]
64536-78-3 | [Synonyms]
CRABTREE CATALYST
CRABTREE'S CATALYST
[Ir(cod)(PCy3)(py)]PF6
CRABTREE PRECURSOR COMPLEX
tricyclohexylphosphinecyclooctadienepyridineiridium PF6
(1,5-cyclooctadiene)(pyrid.)(tric.hexyl-phosphine)irid(I)pf6
1,5-Cyclooctadiene(pyridine)(tricyclohexylphosphine)iridiuM(I)
(TRICYCLOHEXYLPHOSPHINE)(1,5-CYCLOOCTA- DIENE)(PYRIDINE)IRIDIUM(I) PF6
(1,5-CYCLOOCTADIENE)(PYRIDINE)(TRICYCLOHEXYLPHOSPHINE)IRIDIUM(I) PF6, 85%
1,5-Cyclooctadiene-(pyridyl)-(tricyclohexylphosphine)-iridium hexafluoropho
(1,5-Cyclooctadiene)pyridine(tricyclohexylphosphine)iridium hexafluorophosphate
(Tricyclohexyphosphine)(1,5-cyclooctadiene)(pyridine)iridium(I) hexafluorophosphate
(1,5-CYCLOOCTADIENE)(PYRIDINE)(TRICYCLOHEXYLPHOSPHINE)IRIDIUM(I) HEXAFLUOROPHOSPHATE
(TRICYCLOHEXYLPHOSPHINE)(1,5-CYCLOOCTADIENE)(PYRIDINE)IRIDIUM (I) HEXAFLUOROPHOSPHATE
Hexafluorophosphate (tricyclohexyl phosphine) (1,5-cyclooctadiene) (pyridine) iridium
(Tricyclohexylphosphine)(1,5-cyclooctadiene)(pyridine)iridium(I) hexafluorophosphate,98%
(Tricyclohexylphosphine)(1,5-cyclooctadiene)(pyridine)iridium(I) hexafluorophosphate,99%
(1,5-Cyclooctadiene)(pyridine)(tricyclohexylphosphine)iridium(I) Hexafluoro phosphate
(1,5-Cyclooctadiene)(pyridine)(tricyclohexylphosphine)-iridiuM(I) hexafluorophosphate 85%
IRIDIUM(I) HEXAFLUOROPHOSPHATE (1,5-CYCLOOCTADIENE)-(PYRIDINE)-(TRICYCLOHEXYLPHOSPHINE) COMPLEX
(1,5-Cyclooctadiene)(pyridine)(tricyclohexylphosphine)-iridiuM(I) hexafluorophosphate >=99.0% (C)
Crabtrees Catalyst~(Tricyclohexyphosphine)(1,5-cyclooctadiene)(pyridine)iridium(I) hexafluorophosphate
(Tricyclohexylphosphine)(1,5-cyclooctadiene)(pyridine)iridiuM(I)hexafluorophosphate,CRABTREE'S CATALYST
(cycloocta-1,5-diene)pyridyl(tricyclohexyl-phosphine)iridium(I)hexafluorophosphate Crabtree's catalyst
(Tricyclohexylphosphine)(1,5-cyclooctadiene)(pyridine)iridium(I)hexafluorophosphate,99%CRABTREE'SCATALYST
(Tricyclohexylphosphine)(1,5-cyclooctadiene)(pyridine)iridiuM(I) hexafluorophosphate,98% CRABTREE'S CATALYST
Crabtrees catalyst, (1,5-Cyclooctadiene)(pyridine)(tricyclohexylphosphine)-Ir(I) PF6, Iridium(I) hexafluorophosphate (1,5-Cyclooctadiene)-(pyridine)-(tricyclohexylphosphine) complex | [Molecular Formula]
C31H50F6IrNP2 | [MDL Number]
MFCD00075097 | [MOL File]
64536-78-3.mol | [Molecular Weight]
804.89 |
| Chemical Properties | Back Directory | [Appearance]
orange crystals | [Melting point ]
175 °C (dec.)(lit.)
| [density ]
1.67 g/cm3 | [storage temp. ]
2-8°C | [solubility ]
Chloroform (Slightly) | [form ]
Crystalline Powder | [color ]
Orange | [Water Solubility ]
Slightly soluble in acetone, dichloromethane, ethanol and diethyl ether. Insoluble in water. | [Sensitive ]
Moisture Sensitive | [Merck ]
13,2594 |
| Hazard Information | Back Directory | [Chemical Properties]
orange crystals | [Uses]
1,5-Cyclooctadiene(pyridine)(tricyclohexylphosphine)iridium(I) hexafluorophosphate is used as a catalyst for hydrogenation of mono-, di-, tri-, and tetra-substituted substrates and the isomerization and hydroboration of alkenes. It is also used in isotope exchange reactions, especially the direct exchange of a hydrogen atom with its isotopes deuterium and tritium. Carbonyl groups are also known to direct the hydrogenation by the Crabtree catalyst is highly regioselective. | [reaction suitability]
core: iridium
reagent type: catalyst |
| Questions And Answer | Back Directory | [Reaction]
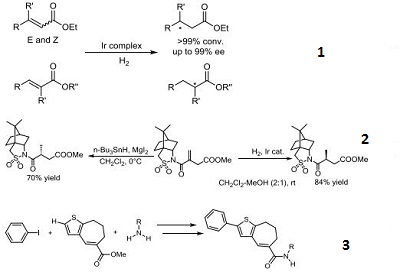
- Iridium catalyst used for the highly enantioselective hydrogenation of α,β-unsaturated esters.
- Iridium catalyst used for the stereoselective catalytic hydrogenation and conjugate reduction of 4methylitaconate derivatives bearing a chiral auxiliary.
- Iridium catalyst used in the synthesis of thiophene-based TAK-779 analogues via C-H arylation.
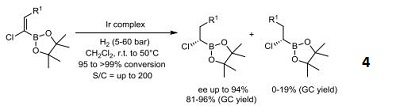
- Iridium catalyst used in the practical synthetic approach to chiral (α-chloroalkyl)boronic esters via an iridiumcatalyzed, chemoselective hydrogenation of chloro-substituted alkenyl boronates.
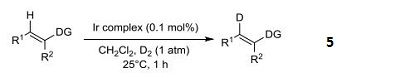
- Iridium catalyst used in the regioselective C-H activation and hydrogen-isotope exchange of non-aromatic unsaturated functionality.
|
|
|