| Identification | More | [Name]
3-Chloropropionyl chloride | [CAS]
625-36-5 | [Synonyms]
3-CHLOROPROPIONIC ACID CHLORIDE
3-CHLOROPROPIONYL CHLORIDE
BETA-CHLOROPROPIONYL CHLORIDE
3-Chloropropanoyl chloride
3-chloro-propanoylchlorid
beta-Chloropropanoyl chloride
beta-Chloropropionic acid chloride
beta-Chloropropionoyl chloride
-Chloropropanoylchloride
Chloropropionyl chloride
Propionyl chloride, 3-chloro-
3-Chloropropionyl chlorode
3-CHLOROPROPIONYL CHLORIDE, TECH.
3-ChloropropionylChloride,~97%
Propanoyl chloride, 3-chloro-
3-CHLOROPROPIONYL CHLORIDE:SS-CHLOROPROPIONLY CHLORIDE
3-Chlorpropionylchlorid
3-CHLOROPROPIONYL CHLORIDE 99%
3-Chloropropionoic acid chloranhydride
b-Chloropropanoyl chloride | [EINECS(EC#)]
210-890-4 | [Molecular Formula]
C3H4Cl2O | [MDL Number]
MFCD00000747 | [Molecular Weight]
126.97 | [MOL File]
625-36-5.mol |
| Chemical Properties | Back Directory | [Appearance]
Clear colorless to dark brown liquid | [Melting point ]
-32 °C
| [Boiling point ]
143-145 °C(lit.)
| [density ]
1.33 g/mL at 25 °C(lit.)
| [vapor pressure ]
10.0 hPa | [refractive index ]
n20/D 1.457(lit.)
| [RTECS ]
UC3934000 | [Fp ]
146 °F
| [storage temp. ]
Flammables area | [solubility ]
Chloroform, Ethyl Acetate | [form ]
liquid
| [color ]
red-brown
| [PH]
<7 (H2O) | [explosive limit]
8.8-20.2%(V) | [Water Solubility ]
reacts | [Sensitive ]
Moisture Sensitive | [BRN ]
635814 | [Stability:]
Moisture Sensitive | [InChIKey]
INUNLMUAPJVRME-UHFFFAOYSA-N | [CAS DataBase Reference]
625-36-5(CAS DataBase Reference) | [NIST Chemistry Reference]
Propanoyl chloride, 3-chloro-(625-36-5) | [EPA Substance Registry System]
625-36-5(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T+ | [Risk Statements ]
R14:Reacts violently with water.
R22:Harmful if swallowed.
R26:Very Toxic by inhalation.
R34:Causes burns.
R35:Causes severe burns.
R10:Flammable. | [Safety Statements ]
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S38:In case of insufficient ventilation, wear suitable respiratory equipment .
S16:Keep away from sources of ignition-No smoking .
S8:Keep container dry . | [RIDADR ]
UN 3390 6.1/PG 1
| [WGK Germany ]
1
| [F ]
21 | [Autoignition Temperature]
460°C | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
I | [HS Code ]
29159080 |
| Hazard Information | Back Directory | [Description]
3-Chloropropionyl chloride is an important bifunctional reagent. It is capable of acylation and possesses a 2-chloro-ethyl fragment (CH2CH2Cl), which can be subjected to nucleophilic substitution and serves as a masked vinyl group. It can be used as a starting material in many reactions to construct a variety of (hetero)cyclic compounds. | [Chemical Properties]
Clear colorless to dark brown liquid | [Uses]
3-Chloropropionyl chloride is a reagent used in the synthesis of Beclamide (B119400), which is a chlorinated benzylpropanamide used as an anticonvulsant drug. It is used in the treatment of tonic-clonic seizyres and has sedative properties. | [Preparation]
3-Chloropropionyl chloride (1) is commercially available and can be prepared from β-propiolactone (2) and thionyl chloride.1 Other standard methods available for the preparation of acyl chlorides can also be applied: the reaction of acrylic acid (3) or 3-chloropropionic acid (4) with thionyl chloride, phosphoryl chloride, phosgene, or phosphorus trichloride.
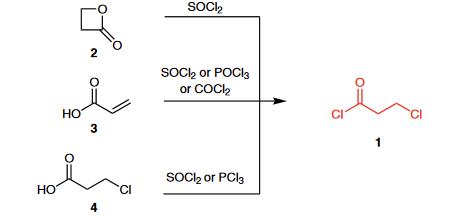 | [Reactions]
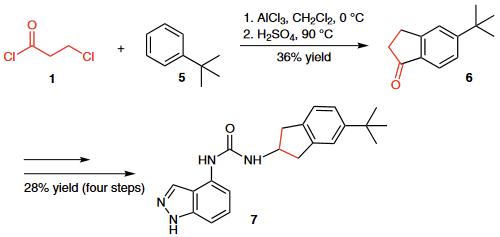
(1) The Friedel¨CCrafts acylation of tert-butylbenzene (5) with 3-chloropropionyl chloride (1) followed by cyclization provid- ed indanone 6, which was further transformed into urea derivative 7, a potent TRPV1 antagonist.
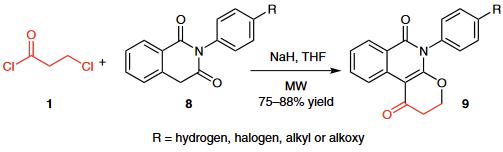
(2) A novel high-yielding one-pot microwave-assisted synthesis of condensed 5-substituted pyranoisoquinoline-1,6-diones 9 from 2-substituted isoquinoline-1,3-diones 8 and 3-chloropropionyl chloride (1) was reported.
(3) Acylation of 2-aminophenol (12) with 3-chloropropionyl chloride (1), followed by cyclization in the presence of polyphosphoric acid (PPA), gave benzoxazole 13, which was further reacted with 4-chlorophenyl-1-piperazine to yield the target benzo[d]oxazole analogue 14, a selective dopamine D4 receptor ligand. | [Flammability and Explosibility]
Nonflammable | [Synthesis]
3-Chloropropionyl chloride is produced by reaction of acrylic acid with hydrogen chloride and phosgene in the presence of, for example, dimethylformamide as catalyst, or by re�action of propiolactone with thionyl chloride. |
|
|