| Identification | More | [Name]
Boc-D-Glutamine | [CAS]
61348-28-5 | [Synonyms]
BOC-D-GLN
BOC-D-GLN-OH
BOC-D-GLUTAMINE
N(ALPHA)-BOC-D-GLUTAMINE
N-ALPHA-T-BOC-D-GLUTAMINE
N-ALPHA-T-BUTOXYCARBONYL-D-GLUTAMINE
NALPHA-(TERT-BUTOXYCARBONYL)-D-GLUTAMIN
NALPHA-(TERT-BUTOXYCARBONYL)-D-GLUTAMINE
N-ALPHA-TERT-BUTYLOXYCARBONYL-D-GLUTAMINE
T-BUTYLOXYCARBONYL-D-GLUTAMINE
N-tert-Butoxycarbonyl-D-glutamine
N(a)-Boc-D-glutamine
NA-T-BOC-D-GLUTAMINE
n(à)-boc-d-glutamine
n(à)-tert-butoxycarbonyl-d-glutamine
N-ALPHA-T-BUTYLOXYCARBONYL-D-GLUTAMINE
Boc-Gln-OH
N(ɑ)-Boc-D-glutamine, 98+%
BOC-D-GLUTAMINE extrapure
(S)-3-(Boc-amino)pentanoic acid 5-amide, Nβ-Boc-L-β-glutamine, Nβ-Boc-L-β-homoasparagine | [EINECS(EC#)]
230-006-0 | [Molecular Formula]
C10H18N2O5 | [MDL Number]
MFCD00190526 | [Molecular Weight]
246.26 | [MOL File]
61348-28-5.mol |
| Chemical Properties | Back Directory | [Melting point ]
117-119°C | [Boiling point ]
389.26°C (rough estimate) | [density ]
1.2430 (rough estimate) | [refractive index ]
1.4550 (estimate) | [storage temp. ]
Store at RT. | [solubility ]
Soluble, 1mmol in 2mL DMF. Soluble in chloroform and methanol. | [form ]
Solid | [pka]
3.84±0.10(Predicted) | [color ]
White to Off-White | [BRN ]
1981311 | [CAS DataBase Reference]
61348-28-5(CAS DataBase Reference) |
| Safety Data | Back Directory | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [HazardClass ]
IRRITANT | [HS Code ]
29241990 |
| Hazard Information | Back Directory | [Chemical Properties]
White solid | [Uses]
N2-Boc-D-glutamine is an N-Boc-protected form of D-Glutamine. D-Glutamine is an unnatural isomer of L-Glutamine that is present in human plasma an is a source of liberated ammonia. D-Glutamine can be synthesized by enzymatic means or can be found in cheeses, wine and vinegars as well. | [Biosynthesis]
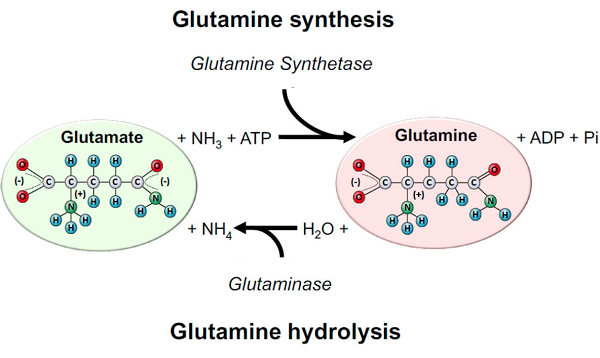
Glutamine is mainly synthesized by the enzyme glutamine synthetase (GS) and hydrolyzed by the enzyme glutaminase (GLS). GS catalyzes glutamine biosynthesis using glutamate and ammonia (NH3) as a source. In this reaction, one ATP is consumed. Glutamate can be donated by many amino acids obtained exogenously (i.e., diet) and/or from endogenous amino acids’ catabolism. On the other hand, GLS is responsible for glutamine hydrolysis to glutamate and ammonium ion (NH4). Almost all cells in the body express GS and GLS, and their predominant expression and activity will dictate if the tissue is more likely to produce or consume glutamine in health and disease[1].
| [reaction suitability]
reaction type: Boc solid-phase peptide synthesis | [References]
[1] Vinicius Cruzat. “Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation.” Nutrients 10 11 (2018).
|
|
|