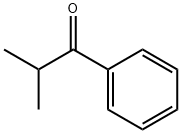
| Identification | More | [Name]
Isobutyrophenone | [CAS]
611-70-1 | [Synonyms]
2-METHYL-1-PHENYL-1-PROPANONE
ALPHA-METHYLPROPIOPHENONE
ISOBUTYROPHENONE
ISOBUTYRYLBENZENE
ISOPROPYL PHENYL KETONE
PHENYL ISOPROPYL KETONE
1-Propanone, 2-methyl-1-phenyl-
1-Propanone,2-methyl-1-phenyl-
2-methyl-1-phenyl-1-propanon
2-methyl-1-phenyl-propan-1-one
2-Methylpropiophenone
Isobutyrophenone,97%
ISOBUTROPHENONE
2-methyl-1-propan-1-one
2-Methyl-1-phenyl-1-propanone, Isobutyrylbenzene
1-Phenyl-2-methyl-1-propanone
2-Methyl-1-phenylpropanone
(2-Methyl-1-oxopropyl)benzene
α-Methylpropiophenone | [EINECS(EC#)]
210-275-0 | [Molecular Formula]
C10H12O | [MDL Number]
MFCD00008917 | [Molecular Weight]
148.2 | [MOL File]
611-70-1.mol |
| Chemical Properties | Back Directory | [Appearance]
clear colorless to very slightly yellow liquid | [Melting point ]
1 °C | [Boiling point ]
217 °C (lit.) | [density ]
0.988 g/mL at 25 °C(lit.)
| [vapor pressure ]
1 mm Hg ( 50 °C)
| [refractive index ]
n20/D 1.517(lit.)
| [Fp ]
184 °F
| [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Chloroform (Sparingly), Methanol (Slightly) | [form ]
Oil | [color ]
Colourless | [Water Solubility ]
immiscible | [BRN ]
774499 | [LogP]
2.73 | [CAS DataBase Reference]
611-70-1(CAS DataBase Reference) | [NIST Chemistry Reference]
Isopropyl phenyl ketone(611-70-1) | [EPA Substance Registry System]
611-70-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [TSCA ]
Yes | [HS Code ]
29143900 |
| Hazard Information | Back Directory | [Chemical Properties]
clear colorless to very slightly yellow liquid | [Uses]
Isobutyrophenone was used as an internal standard in the determination of hydroxyzine hydrochloride and benzyl alcohol in injection solutions. It was also used in the preparation of alpha-hydroxyisobutyrophenone. | [Uses]
Isobutyrophenone is used as an internal standard in the determination of hydroxyzine hydrochloride and benzyl alcohol in injection solutions. It is also used in the preparation of alpha-hydroxyisobutyrophenone. Isobutyrophenone is employed as photosensitizer intermediate. It can react with bromoacetic acid ethyl ester to get 3-hydroxy-4-methyl-3-phenyl-valeric acid ethyl ester. | [Preparation]
Isobutyrophenone synthesis: Benzene underwent a Friedel–Crafts acylation with isobutyryl chloride to synthesize isobutyrophenone. The reaction was performed using excess benzene as a replacement for a typical solvent and heat to reflux (88 °C) for a period of 3 hours. Any unreacted benzene was recovered after the reaction during the distillation process. A 66 % yield of isobutyrophenone was obtained from the solar synthesis, compared to a 44 % yield from an in-lab, electrical heating analysis.
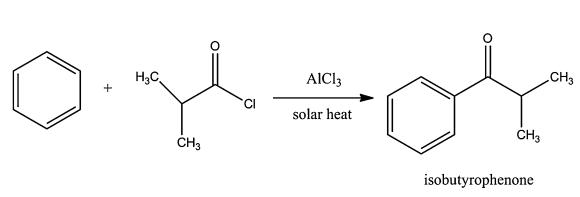 | [Synthesis Reference(s)]
Journal of the American Chemical Society, 71, p. 1061, 1949 DOI: 10.1021/ja01171a084
The Journal of Organic Chemistry, 32, p. 404, 1967 DOI: 10.1021/jo01288a032
Tetrahedron Letters, 14, p. 2491, 1973 | [Flammability and Explosibility]
Nonflammable |
|
|