| Identification | More | [Name]
9-PHENYLANTHRACENE | [CAS]
602-55-1 | [Synonyms]
9-PHENYLANTHRACENE
anthracene,9-phenyl-
Phenylanthracene,98%
9-PHENYLANTHRACENE OEKANAL, 250 MG
9-Phenylanthracene97%
9-Phenylanthracene 97% | [EINECS(EC#)]
210-019-8 | [Molecular Formula]
C20H14 | [MDL Number]
MFCD00001252 | [Molecular Weight]
254.33 | [MOL File]
602-55-1.mol |
| Chemical Properties | Back Directory | [Appearance]
Pale Yellow Plates | [Melting point ]
153-155 °C (lit.) | [Boiling point ]
417 °C (lit.) | [density ]
1.1180 (estimate) | [refractive index ]
1.7040 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Chloroform (Slightly), Ethyl Acetate (Slightly) | [form ]
Solid | [color ]
Off-white to pale brown | [Sensitive ]
Light Sensitive | [BRN ]
1910570 | [CAS DataBase Reference]
602-55-1(CAS DataBase Reference) | [EPA Substance Registry System]
Anthracene, 9-phenyl- (602-55-1) |
| Safety Data | Back Directory | [Hazard Codes ]
N | [Risk Statements ]
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment . | [Safety Statements ]
S60:This material and/or its container must be disposed of as hazardous waste .
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [RIDADR ]
UN 3077 9/PG 3
| [WGK Germany ]
3
| [HazardClass ]
9 | [HS Code ]
29029090 |
| Hazard Information | Back Directory | [Chemical Properties]
Pale Yellow Plates | [Uses]
9-Phenylanthracene is used to synthesize organic light-emitting material intermediates. | [Synthesis Reference(s)]
Journal of the American Chemical Society, 79, p. 393, 1957 DOI: 10.1021/ja01559a042 | [Purification Methods]
Chromatograph it on alumina in *C6H6 and recrystallise it from AcOH or toluene. [Beilstein 5 H 725, 5 II 639, 5 III 2462.] |
| Questions And Answer(Q&A) | Back Directory | [Preparation]
Phenylboronic acid (2.13 g, 17.49 mmol), 9- bromoanthracene (3 g, 11.66 mmol), Pd(PPh3)4 (140 mg, 0.12 mmol), K2CO3 (16 mL, 2 M), and ethanol (8 mL) were mixed in a 100 mL flask containing anhydrous toluene (32 mL). The mixture was refluxed for 48 h under nitrogen. After cooled to room temperature, the reaction mixture was quenched with dilute hydrochloric acid solution and extracted with CH2Cl2. The organic extracts were dried over anhydrous MgSO4 and concentrated by rotary evaporation. The crude product was further purified by silica gel column chromatography (CH2Cl2/petroleum ether (1:4,v/v)) to get a white powder (2.43 g, 81.9 %). 1H NMR (500 MHz, CDCl3) δ 8.53 (s, 1H), 8.08 (d, J = 8.5 Hz, 2H), 7.70 – 7.67 (m, 2H), 7.63 – 7.54 (m, 3H), 7.50 – 7.45 (m, 4H), 7.37 (ddd, J = 8.7, 6.5, 1.2 Hz, 2H). EI-MS (m/z): Calculated for C20H14: 254.33. Found [M+]: 253.02.
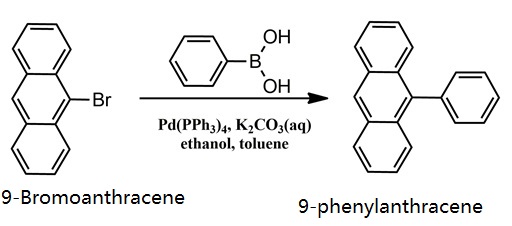
Synthesis of 9-phenylanthracene
|
|
|