| Identification | More | [Name]
1,3-CYCLOHEXADIENE | [CAS]
592-57-4 | [Synonyms]
1,2-DIHYDROBENZENE
1,3-CYCLOHEXADIENE
DIHYDROBENZENE
l,3-Cyclohexadiene
Cyclohexa-1,3-diene
1,3-Cyclohexadiene (stabilized with BHT)
Cyclohexadiene
1,3-CYCLOHEXADIENE, STAB.
1,3-Cyclohexadiene,96%
1,3-Cyclohexadiene, stabilized, 96%
1,3-Cyclohexadiene, 96%, stab.
1,3-Cyclohexadiene, 96%, stabilized
1,3-Cyclohexanediene
1,3-Cyclohexadiene, 96%, stab. with 0.1% BHT | [EINECS(EC#)]
209-764-1 | [Molecular Formula]
C6H8 | [MDL Number]
MFCD00001532 | [Molecular Weight]
80.13 | [MOL File]
592-57-4.mol |
| Chemical Properties | Back Directory | [Appearance]
clear colorless to light yellow liquid | [Melting point ]
-98 °C | [Boiling point ]
80 °C (lit.) | [density ]
0.841 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.474(lit.)
| [Fp ]
−2 °F
| [storage temp. ]
2-8°C
| [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Liquid | [color ]
Clear colorless to light yellow | [Water Solubility ]
Slightly miscible with methanol. Immiscible with water. | [BRN ]
506024 | [Dielectric constant]
2.6600000000000001 | [Stability:]
Light Sensitive | [LogP]
2.470 | [CAS DataBase Reference]
592-57-4(CAS DataBase Reference) | [EPA Substance Registry System]
1,3-Cyclohexadiene (592-57-4) |
| Safety Data | Back Directory | [Hazard Codes ]
F | [Risk Statements ]
R11:Highly Flammable.
R10:Flammable. | [Safety Statements ]
S9:Keep container in a well-ventilated place .
S16:Keep away from sources of ignition-No smoking .
S29:Do not empty into drains .
S33:Take precautionary measures against static discharges . | [RIDADR ]
UN 3295 3/PG 2
| [WGK Germany ]
3
| [RTECS ]
GU4702350
| [F ]
9 | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
II | [HS Code ]
29021990 |
| Hazard Information | Back Directory | [Description]
1,3-Cyclohexadiene is an organic compound with the formula (C2H4)(CH)4. It is a colorless, flammable liquid. It is a useful diene for proteomics research. | [Chemical Properties]
clear colorless to light yellow liquid | [Reactions]
1,3-Cyclohexadiene can undergo:
- C-C coupling with aromatic alcohols via iridium-catalyzed hydrogen auto-transfer and with aldehydes via transfer hydrogenation mediated by isopropanol to form carbonyl addition products.
- Living anionic polymerization with n-BuLi/TMEDA system to form polycyclohexadiene.
- Platinum-catalyzed silaboration to form (1R,4S)-1-(dimethylphenylsilyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-cyclohexene.
- Aerobic palladium-catalyzed 1,4-diacetoxylation in the presence of cobalt tetra(hydroquinone)porphyrin as an electron transfer reagent.
| [Uses]
1,3-Cyclohexadiene can undergo:
- C-C coupling with aromatic alcohols via iridium-catalyzed hydrogen auto-transfer and with aldehydes via transfer hydrogenation mediated by isopropanol to form carbonyl addition products.
- Living anionic polymerization with n-BuLi/TMEDA system to form polycyclohexadiene.
- Platinum-catalyzed silaboration to form (1R,4S)-1-(dimethylphenylsilyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-cyclohexene.
- Aerobic palladium-catalyzed 1,4-diacetoxylation in the presence of cobalt tetra(hydroquinone)porphyrin as an electron transfer reagent.
| [Uses]
1,3-Cyclohexadiene can undergo:
C-C coupling with aromatic alcohols via iridium-catalyzed hydrogen auto-transfer and with aldehydes via transfer hydrogenation mediated by isopropanol to form carbonyl addition products.
Living anionic polymerization with n-BuLi/TMEDA system to form polycyclohexadiene.
Platinum-catalyzed silaboration to form (1R,4S)-1-(dimethylphenylsilyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-cyclohexene.
Aerobic palladium-catalyzed 1,4-diacetoxylation in the presence of cobalt tetra(hydroquinone)porphyrin as an electron transfer reagent. | [Uses]
1,3-Cyclohexadiene is used as a hydrogen donor in transfer hydrogenation. It is used in the conversion to benzene. It is useful in the study of proteomics research. | [Definition]
ChEBI: Cyclohexa-1,3-diene is a cyclohexadiene. | [Preparation]
1,3-Cyclohexadiene has been prepared by dehydration of cyclohexen-3-ol, by pyrolysis at 540° of the diacetate of cyclohexane-1,2-diol, by dehydrobromination with quinoline of 3-bromocyclohexene, by treating the ethyl ether of cyclohexen-3-ol with potassium bisulfate, by heating cyclohexene oxide with phthalic anhydride, by treating cyclohexane-1,2-diol with concentrated sulfuric acid, by treatment of 1,2-dibromocyclohexane with tributylamine, with sodium hydroxide in ethylene glycol, and with quinoline, and by treatment of 3,6-dibromo-cyclohexene with sodium.
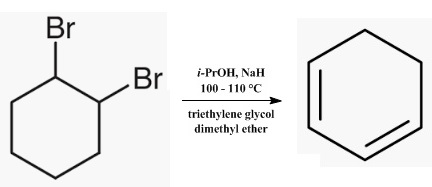
| [Purification Methods]
Distil the diene from NaBH4 or Na under N2 and collect it in a trap cooled in Dry Ice. It is highly flammable. [Marvel & Martell, J Am Chem Soc 81 450 1959, Beilstein 5 IV 382.] |
|
|