| Identification | Back Directory | [Name]
OLIGOMYCIN A | [CAS]
579-13-5 | [Synonyms]
Oli
MCH 32
CS-1898
mycin A
AOligoMyci
OLIGOMYCIN A
OLIGOMYCIN COMPLEX
Oligomycin A, >=98%
OLIGOMYCIN A USP/EP/BP
Oligomycin A
MCH 32
Oligomycin A from Streptomyces
OLIGOMYCIN, STREPTOMYCES DIASTATOCHROMOGENES
OLIGOMYCIN A, STREPTOMYCES DIASTATOCHROMOGENES
Oligomycin A fromStreptomyces diastatochromogenes
Oligomycin A, 99%, from Streptomyces diastatochromogenes
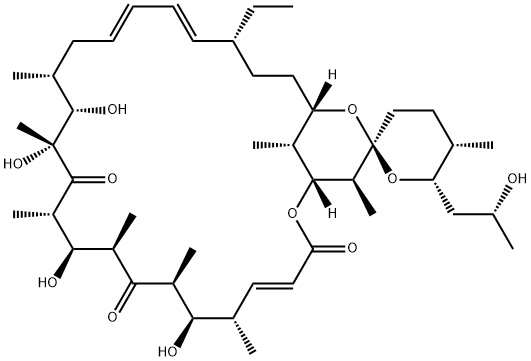
(1R,4E,5'S,6S,6'S,7R,8S,10R,11R,12S,14R,15S,16R,18E,20E,22R,25S,27R,28S,29R)-22-ethyl-7,11,14,15-tetrahydroxy-6'-[(2R)-2-hydroxypropyl]-5',6,8,10,12,14,16,28,29-nonaMethyl-3',4',5',6'-tetrahydro-3H,9H,13H-spiro[2,26-dioxabicyclo[23.3.1]nonaco
Spiro[2,26-dioxabicyclo[23.3.1]nonacosa-4,18,20-triene-27,2'-[2H]pyran]-3,9,13-trione,22-ethyl-3',4',5',6'-tetrahydro-7,11,14,15-tetrahydroxy-6'-[(2R)-2-hydroxypropyl]-5',6,8,10,12,14,16,28,29-nonamethyl-,(1R,2'R,4E,5'S,6S,6'S,7R,8S,10R,11R,12S,14R,15S,16
Spiro[2,26-dioxabicyclo[23.3.1]nonacosa-4,18,20-triene-27,2'-[2H]pyran]-3,9,13-trione, 22-ethyl-3',4',5',6'-tetrahydro-7,11,14,15-tetrahydroxy-6'-[(2R)-2-hydroxypropyl]-5',6,8,10,12,14,16,28,29-nonamethyl-, (1R,2'R,4E,5'S,6S,6'S,7R,8S,10R,11R,12S,14R,15S,16R,18E,20E,22R,25S,28S,29R)-
(1R,4E,5'S,6S,6'S,7R,8S,10R,11R,12S,14R,15S,16R,18E,20E,22R,25S,27R,28S,29R)-22-ethyl-7,11,14,15-tetrahydroxy-6'-[(2R)-2-hydroxypropyl]-5',6,8,10,12,14,16,28,29-nonamethyl-3',4',5',6'-tetrahydro-3H,9H,13H-spiro[2,26-dioxabicyclo[23.3.1]nonacosa-4,18,20-triene-27,2'-pyran]-3,9,13-trione | [EINECS(EC#)]
209-437-3 | [Molecular Formula]
C45H74O11 | [MDL Number]
MFCD00065705 | [MOL File]
579-13-5.mol | [Molecular Weight]
791.06 |
| Chemical Properties | Back Directory | [Melting point ]
150-151℃ | [Boiling point ]
886.3±65.0 °C(Predicted) | [density ]
1.14 | [storage temp. ]
Sealed in dry,Store in freezer, under -20°C | [solubility ]
Soluble in DMSO, ethanol or acetone | [form ]
powder | [pka]
12.52±0.70(Predicted) | [color ]
white to off-white | [Merck ]
13,6902 | [BRN ]
5702132 | [InChIKey]
MNULEGDCPYONBU-WMBHJXFZSA-N |
| Hazard Information | Back Directory | [Description]
Oligomycins are macrolides created by Streptomyces species that can be toxic to other organisms. Different oligomycin isomers are highly specific for the disruption of mitochondrial metabolism. Oligomycin A, a dominant analog of the isomers, is an inhibitor of mitochondrial F1FO ATP synthase that induces apoptosis in a variety of cell types (average GI50 = 270 nM).1,2,3 Oligomycin A exhibits antifungal, antitumor, and nematocidal activities, but has poor solubility in water and other biocompatible solvents, which limits its clinical application.4 | [Chemical Properties]
White powder | [Uses]
Oligomycin A is a macrolide with fungicidal activity isolated from Streptomyces species. | [Uses]
Oligomycin A is the dominant analogue of a class macrocyclic lactones isolated from selected strains of Streptomyces sp.. Oligomycin A is an inhibitor of mitochondrial F1F0-ATPase. It induces apoptosis in a variety of cell types, makes cells more susceptible to cell death, and also leads to a switch in the death mode from apoptosis to necrosis. Oligomycin A exhibits a broad biological profile including antifungal, antitumour and nematocidal activities. | [Uses]
Oligomycin A is the dominant analogue of a class of macrocyclic lactones isolated from selected strains of Streptomyces sp.. Oligomycin A is an inhibitor of mitochondrial F1F0-ATPase. It induces apoptosis in a variety of cell types, makes cells more susceptible to cell death, and also leads to a switch in the death mode from apoptosis to necrosis. Oligomycin A exhibits a broad biological profile including antifungal, antitumor and nematocidal activities. | [storage]
-20°C | [References]
[1]. jastroch m, divakaruni as, mookerjee s, et al. mitochondrial proton and electron leaks. essays biochemistry, 2010, 47:53-67.
[2]. shchepina la, pletjushkina oy, avetisyan av, et al. oligomycin, inhibitor of the f-0 part of h+-atp-synthase, suppresses the tnf-induced apoptosis. oncogene, 2002, 53: 8149-8157.
[3]. salomon ar, voehringer dw, herzenberg la, et al. understanding and exploiting the mechanistic basis for selectivity of polyketide inhibitors of f0f1-atpase. proceedings of the national academy of sciences of the united states of america, 2000, 97(26): 14766-14771.
[4]. alexander r, adina v, ivan b, et al. overcoming intrinsic multi-drug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling jarid1bhigh cells. cancer cell, 2013, 23(6): 811-825. |
|
|