| Identification | Back Directory | [Name]
2-IMIDAZOL-1-YL-ETHYLAMINE DIHYDROCHLORIDE | [CAS]
5739-10-6 | [Synonyms]
2-ethyl imidazoleamine
1-(2-Aminoethyl)imidazole
1H-Imidazole-1-ethanamine
N-(2-Aminoethyl)imidazole
2-Imidazol-1-yl-ethylamine1500
1H-Imidazole-1-ethanamine 2HCl
2-(1H-Imidazol-1-yl)ethanamine
2-IMIDAZOL-1-YL-ETHYLAMINE 2HCL
2-IMIDAZOL-1-YL-ETHYLAMINE DIHYDROCHLORIDE
2-(1H-imidazol-1-yl)ethanamine(SALTDATA: 2HCl)
[2-(1H-imidazol-1-yl)ethyl]amine hydrochloride
2-(1H-iMidazol-1-yl)ethan-1-aMine dihydrochloride | [Molecular Formula]
C5H9N3 | [MDL Number]
MFCD06798214 | [MOL File]
5739-10-6.mol | [Molecular Weight]
111.15 |
| Chemical Properties | Back Directory | [Boiling point ]
283℃ | [density ]
1.15 | [Fp ]
125℃ | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [pka]
7.51±0.10(Predicted) |
| Hazard Information | Back Directory | [Uses]
2-(1H-Imidazol-1-yl)ethanamine is an intermediate for the preparation of selective tumor inhibitor bis(nitroimidazolyl)alkanecarboxamide hypoxia. | [Synthesis]
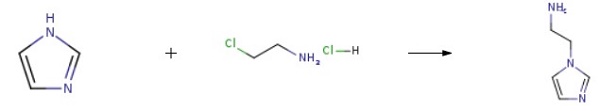
Imidazole (8.10 g, 119 mmol), 2-chloroethylamine monohydrochloride (15.2 g, 131 mmol), tetrabutylammonium hydrogensulfate (1.62 g, 4.8 mmol), and sodium hydroxide (17.1 g, 428 mmol) were combined with 100 ml acetonitrile and heated under reflux for 21 h. The reaction mixture was cooled and filtered. The filtrate was concentrated to a pale yellow oil. Flash chromatography (silica gel, gradient elution of acetonitrile to 9:1 acetonitrile/NH4OH) afforded 4.52 g (34%) of 2-(1H-Imidazol-1-yl)ethanamine as a pale yellow oil.
|
|
|