| Identification | More | [Name]
Urea | [CAS]
57-13-6 | [Synonyms]
AKOS BBS-00004448
CARBAMIDE
CARBIMIDE
CARBONYLDIAMIDE
Carbonyl diamine
HARNSTOFF
ISOUREA
UREA
UREA FERTILIZER
UREA HYDROCHLORIDE
UREA, PRILLED
UREUM
(NH2)2CO
ai3-01202
Alphadrate
amideofcarbonicacid
Aqua Care
aquacare
Aquacare HP
aquacarehp | [EINECS(EC#)]
200-315-5 | [Molecular Formula]
CH4N2O | [MDL Number]
MFCD00008022 | [Molecular Weight]
60.06 | [MOL File]
57-13-6.mol |
| Chemical Properties | Back Directory | [Appearance]
Urea is a white crystalline so lid. | [Melting point ]
132-135 °C(lit.)
| [Boiling point ]
332.48°C (estimate) | [bulk density]
720-760kg/m3 | [density ]
1.335 g/mL at 25 °C(lit.)
| [vapor pressure ]
<0.1 hPa (20 °C) | [refractive index ]
n20/D 1.40
| [storage temp. ]
2-8°C
| [solubility ]
H2O: 8 M at 20 °C
| [form ]
powder
| [pka]
0.10(at 25℃) | [color ]
white
| [Specific Gravity]
1.335 | [Odor]
almost odorless | [PH]
8.0-10.0 (20℃, 8M in H2O) | [Stability:]
Substances to be avoided include strong oxidizing agents. Protect from moisture. | [biological source]
synthetic | [Water Solubility ]
1080 g/L (20 ºC) | [λmax]
λ: 260 nm Amax: 0.03
λ: 280 nm Amax: 0.02 | [Merck ]
14,9867 | [BRN ]
635724 | [Dielectric constant]
3.5(Ambient) | [InChIKey]
XSQUKJJJFZCRTK-UHFFFAOYSA-N | [LogP]
-1.660 (est) | [Uses]
urea is incorporated into cosmetics for a variety of purposes, including moisturizing, desquamating, anti-microbial, and buffering. urea is regarded as a “true” moisturizer rather than a humectant because it attracts and retains moisture in the corneum layer. It facilitates the natural exfoliation of keratinocytes given its ability to dissolve intercellular cement in the corneum layer. Through its anti-microbial properties that inhibit the growth of micro-organisms in a product, urea can also be part of a larger preservative system. This ingredient’s buffering action is attributed to its ability to regulate the hydrolipid mantle. In addition, urea is found to enhance the penetration and absorption of other active ingredients, relieve itchiness, and help leave the skin feeling soft and supple. Anti-inflammatory, anti-septic, and deodorizing actions allow it to protect the skin’s surface against negative changes and help maintain healthy skin. Studies show that urea does not induce photoallergy, phototoxicity, or sensitization. The safest concentration of use in skin care preparations is between 2 and 8 percent. High concentrations of urea seem to be unstable when incorporated into skin care preparations and can also cause irritation. Acidic urea solutions can produce burning or stinging sensations. | [CAS DataBase Reference]
57-13-6(CAS DataBase Reference) | [NIST Chemistry Reference]
Urea(57-13-6) | [EPA Substance Registry System]
57-13-6(EPA Substance) | [Absorption]
≤0.06 at 260nm at 5M
≤0.06 at 280nm at 5M |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
Not regulated | [WGK Germany ]
1
| [RTECS ]
YR6250000
| [TSCA ]
Yes | [HS Code ]
31021010 | [Safety Profile]
Moderately toxic by
intravenous and subcutaneous routes.
Human reproductive effects by
intraplacental route: ferthty effects.
Experimental reproductive effects. Human
mutation data reported. A human skin
irritant. Questionable carcinogen with
experimental carcinogenic and
neoplastigenic data. Reacts with sodium
hypochlorite or calcium hypochlorite to
form the explosive nitrogen trichloride.
Incompatible with NaNO2, P2Cl5, nitrosyl
perchlorate. Preparation of the 15N-labeled
urea is hazardous. When heated to
decomposition it emits toxic fumes of NOx. | [Hazardous Substances Data]
57-13-6(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 8471 mg/kg LD50 dermal Rat 8200 mg/kg |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Acetic acid-->Ammonia-->Carbon dioxide-->Glycine-->Diethanolamine-->PETROLEUM ETHER-->METALLURGICAL COKE-->HEAVY CUT RESIDUE OIL-->Vanadium(V) oxide-->Liquefied petroleum ges-->AMMONIUM CARBAMATE | [Preparation Products]
2-HYDROXY-4-PHENYLPYRIMIDINE-5-CARBOXYLIC ACID-->ETHYL 2-HYDROXYPYRIMIDINE-5-CARBOXYLATE-->rubber latex 104T/C-->Cymoxanil-->4,5-dihydroxy-1,3-bis(hydroxymethyl)imidazolidin-2-one-->Tableware cleaner-->Carbachol-->Biuret-->Reactive Black KN-BN-->DICHLOROISOCYANURIC ACID-->5-CARBETHOXYURACIL-->Pigment Yellow 65-->2,6-Dichloro-4,8-dipiperidinopyrimidino[5,4-d]pyrimidine-->biodegrddable finishing agent for fabric-->2,4,6,8-Tetrahydroxy-Pyrimido-(5,4D)Pyrimidine-->DIRECT FAST BLACK G-->Amino moulding plastic-->Water flush fertilizer-->Nitrofurazone-->ALLYLUREA-->flame retardane ZR-01-->1-PHENYLSEMICARBAZIDE-->2,4-Dichlorothieno[3,2-d]pyrimidine-->1-(1,3-DIHYDRO-1-OXOISOBENZOFURAN-3-YL)UREA-->2,4-DICHLOROTHIENO[3,2-D]PYRIMIDINE-->6-Aminouracil-->ureaformaldelyde resin UF-->synthetic carbamider ring tanning agent No.1-->5-(sec-pentyl)barbituric acid-->synthetic tanning agent PNC-->Tanning agent for white leather-->1-Methyl-1-nitrosourea-->N,N''-(isobutylidene)diurea-->2,4-DIHYDROXYTHIENO[3,2-D]PYRIMIDINE-->1-(2-Aminoethyl)imidazolidin-2-one-->BIUREA-->5,6-DIHYDROURACIL-->DIHYDROTHYMINE-->3-Hydroxy-1-phenyl-1,2,4-triazole-->Urea hydrogen peroxide |
| Hazard Information | Back Directory | [General Description]
Solid odorless white crystals or pellets. Density 1.335 g /cc. Noncombustible. | [Reactivity Profile]
UREA(57-13-6) is a weak base. Reacts with hypochlorites to form nitrogen trichloride which explodes spontaneously in air [J. Am. Chem. Soc. 63:3530-32]. Same is true for phosphorus pentachloride. UREA(57-13-6) reacts with azo and diazo compounds to generate toxic gases. Reacts with strong reducing agents to form flammable gases (hydrogen). The heating of improper stoichiometric amounts of UREA(57-13-6) and sodium nitrite lead to an explosion. Heated mixtures of oxalic acid and UREA(57-13-6) yielded rapid evolution of gases, carbon dioxide, carbon monoxide and ammonia (if hot, can be explosive). Titanium tetrachloride and UREA(57-13-6) slowly formed a complex during 6 weeks at 80°C., decomposed violently at 90°C., [Chem. Abs., 1966, 64, 9219b]. UREA(57-13-6) ignites spontaneously on stirring with nitrosyl perchlorate, (due to the formation of the diazonium perchlorate). Oxalic acid and UREA(57-13-6) react at high temperatures to form toxic and flammable ammonia and carbon monoxide gasses, and inert CO2 gas [Von Bentzinger, R. et al., Praxis Naturwiss. Chem., 1987, 36(8), 41-42]. | [Air & Water Reactions]
Water soluble. | [Health Hazard]
May irritate eyes. | [Potential Exposure]
Urea is used in ceramics, cosmetics,
paper processing; resins, adhesives, in animal feeds; in the
manufacture of isocyanurates; resins, and plastics; as a stabilizer
in explosives; in medicines; anticholelithogenic, and
others. | [Fire Hazard]
Behavior in Fire: Melts and decomposes, generating ammonia. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit. | [Incompatibilities]
Violent reaction with strong oxidizers,
chlorine, permanganates, dichromates, nitrites, inorganic
chlorides; chlorites, and perchlorates. Contact with hypochlorites
can result in the formation of explosive compounds. | [Description]
Urea is a stable highly water-soluble compound
of high nitrogen content (47%), with good storage
properties that make it the most commonly used nitrogen
fertilizer. The synthesis process has remained essentially
unchanged since it was first developed by the BASF
Corporation in 1922. In this process, liquid ammonia
is reacted with carbon dioxide to produce ammonium
carbamate, which is then dehydrated to form urea. The
reactions are:
2NH3 + CO2 ===? NH2·CO2·NH4
NH2·CO2·NH4 ===? (NH2)2CO + H2O
| [Waste Disposal]
Controlled incineration in
equipment containing a scrubber or thermal unit to reduce
nitrogen oxide emissions. | [Definition]
A
white crystalline compound made from
ammonia and carbon dioxide. It is used in
the manufacture of urea–formaldehyde
(methanal) resins. Urea is the end product
of metabolism in many animals and is present in urine. | [Definition]
ChEBI: A carbonyl group with two C-bound amine groups. | [Indications]
Urea-containing preparations have a softening and moisturizing effect on the stratum
corneum and, at times, may provide good therapy for dry skin and the pruritus
associated with it. They appear to have an antipruritic effect apart from their hydrating
qualities. Urea compounds disrupt the normal hydrogen bonds of epidermal
proteins; therefore, their effect in dry hyperkeratotic diseases such as ichthyosis
vulgaris and psoriasis is not only to make the skin more pliable but also to help
remove adherent scales. Lactic acid also has a softening and moisturizing effect on
the stratum corneum.
Urea 40% ointment may be useful in removing hypertrophic or dystrophic
psoriatic nails. Subsequent topical therapy to the denuded nail bed and proximal
nail fold may result in regrowth of ‘‘normal’’ nails in half of those treated. | [Preparation]
All current processes for the manufacture of urea are based on the reaction of
ammonia and carbon dioxide to form ammonium carbamate which is
simultaneously dehydrated to urea:

The dehydration of ammonium carbamate is appreciable only at temperatures
above the melting point (about 150??C) and this reaction can only
proceed if the combined partial pressure of ammonia and carbon dioxide
exceeds the dissociation pressure of the ammonium carbamate (about
10 MPa at 160??C and about 30 MPa at 200??C). Thus commercial processes
are operated in the liquid phase at 160-220??C and 18-35 MPa (180-350
atmospheres). Generally, a stoichiometric excess of ammonia is employed,
molar ratios of up to 6: 1 being used. The dehydration of ammonium
carbamate to urea proceeds to about 50-65% in most processes. The reactor
effluent therefore consists of urea, water, ammonium carbamate and the
excess of ammonia. Various techniques are used for separating the components.
In one process the effluent is let down in pressure and heated at about
155??C to decompose the carbamate into ammonia and carbon dioxide. The
gases are removed and cooled. All the carbon dioxide present reacts with the
stoichiometric amount of ammonia to re-form carbamate, which is then
dissolved in a small quantity of water and returned to the reactor. The
remaining ammonia is liquefied and recycled to the reactor. Fresh make-up
ammonia and carbon dioxide are also introduced into the reactor. Removal of
ammonium carbamate and ammonia from the reactor effluent leaves an
aqueous solution of urea. The solution is partially evaporated and then urea is
isolated by recrystallization. Ammonium carbamate is very corrosive and at one time it was necessary to use silver-lined equipment but now satisfactory
alloy steel plant is available. | [Brand name]
Ureaphil (Hospira). | [Biological Functions]
The use of urea (Ureaphil, Urevert) has declined in
recent years owing both to its disagreeable taste and to
the increasing use of mannitol for the same purposes.
When used to reduce cerebrospinal fluid pressure, urea
is generally given by intravenous drip. Because of its potential
to expand the extracellular fluid volume, urea is
contraindicated in patients with severe impairment of
renal, hepatic, or cardiac function or active intracranial
bleeding. | [Agricultural Uses]
Fertilizer, Fungicide: Used in fertilizers and animal feeds, as a fungicide,
in the manufacture of resins and plastics, as a stabilizer
in explosives and in medicines, and others. Urea is used
to protect against frost and is used in some pesticides as
an inert ingredient as a stabilizer, as an inhibitor and as an
intensifier for herbicides. Registered for use in EU countries
. Registered for use in the U.S. | [Agricultural Uses]
Urea, CO(NH2)2, also referred to as carbamide, is a
white, crystalline, organic, water-soluble fertilizer. It
contains around 46 % nitrogen, the highest N percentage
any solid fertilizer can have.
Apart from its major use as a fertilizer, urea is also
employed in the manufacture of paints, glues, plastics,
paper, textiles, feed and weed control chemicals as well
as a source of non-protein nitrogen.
Urea is an acceptable fertilizer for rice and
preferable to nitrates for flooded rice because of the
reduction of nitrates to N,O and/or nitrogen (in
anaerobic conditions) which is lost to the atmosphere.
Also, rice can utilize the ammonium form of nitrogen
efficiently. Hydrolysis and nitrification (in aerobic
conditions) are rapid in tropical, sub-tropical and warm
climates.
Urea can thus be used efficiently but its use requires a
better understanding than that required for other
inorganic salts. It is applied to flooded soil three times: at
the time of planting, tillering and panicle development.
Similar to other nitrogenous fertilizers, urea promotes
the growth of both weeds and crops. Urea solution after
evaporation in vacuum evaporators, can be finally spraydried
into pellets or prills. When protected from moisture
(to which it is susceptible), urea is non-caking, freeflowing
and suitable for storage and handling.
However, the benefits of urea outweigh its
disadvantages. Insofar as the weed growth is concerned,
effective methods should be devised to minimize it to a
manageable level.
Urea is converted rapidly to ammonia by hydrolysis
in the soil via the ammonium carbonate formation route,
the latter being unstable (decomposing to ammonia and
carbon dioxide). Urea is not as quick acting as
ammonium nitrate because the nitrifying bacteria require
a few days of warm and moist soil conditions to convert
ammonia to the nitrate form. The formation of
ammonium ion is slightly acidic in its ultimate reaction
with the soil.
Urea is decomposed by the enzyme urease and a part
of urea is lost as gaseous nitrogen. The time between urea
application and the first availability of water to the soil is
important, as also the temperature, because the enzyme is
less reactive in cold than at high temperature (25 to
30°C). Prevention and retardation of the hydrolytic
action of urease is important following the addition of
urea to soil. This may help to avoid difficulties associated
with ammonia formation and alkalization.
Many substances are urease inhibitors, but very few
meet the rather specific requirements of being (a)
effective at low concentrations, (b) relatively non-toxic to
higher forms of life, (c) inexpensive, and (d) compatible
with urea.
Urea can be sprayed on leaves and can also be mixed
with insecticides or herbicides for soil application. A
urea-ammonium nitrate mixture with herbicide is also
used for weed control.
Urea, although an excellent fertilizer, suffers from
the following drawbacks: (i) When applied to a bare soil
surface, urea hydrolyzes rapidly and loses a significant
quantity of ammonia by volatilization. Such losses vary
from soil to soil and are greater for urea in a pellet form
rather than in a solution form. Burning residues on the
field is suggested as a practical means to control the
ammonia loss because the burning reduces the
concentration of the enzyme urease in plants. (ii) Rapid
hydrolysis of urea in soils can cause injury to the
seedlings by ammonia, if large quantities of the fertilizer
are placed too close to the seeds. (iii) The fertilizer grade
urea may contain toxic biuret which is formed during
urea manufacture by an excessive temperature rise. A
large concentration of biuret in urea ( > 2 %) causes injury to plants.
Feed-grade urea is sometimes referred to by the
number 262 which is the product of its nitrogen content
(42%) multiplied by 6.25, the latter being the factor used
by chemists to convert nitrogen to its protein equivalent.
Urea is sometimes phytotoxic when placed close to
seeds or seedlings. The phytotoxicity is caused by high
local concentrations of ammonia during the hydrolysis
stage or by accumulation of nitrite during the nitrification
step. Another possible cause is the presence of biuret
impurity in urea.
The whole series of urea-formaldehyde
compounds, ranging from soluble to completely water-insoluble,
are produced by reacting urea with
formaldehyde in different ratios. The fertilizer grade
contains a minimum of 35% nitrogen, largely water-insoluble
but in a gradually available form. The
suitability of these compounds as fertilizers also depends
on the quantity and quality of cold-water-insoluble
nitrogen. The solubility reflects the rate at which the
nitrogen becomes available. Formaldehyde-treated urea
seems to be more waterproof and less subject to
dissolution by light showers or heavy dew.
In addition to the marked improvements in the size,
strength and density of granular urea, urea has a number
of good characteristics compared to ammonium nitrate.
These include its (a) lesser tendency to stick and cake than
ammonium nitrate, (b) insensitivity to fire and explosion,
and (c) resistance to corrosion during handling and
A popular urea-formaldehyde product in the USA
contains 38% nitrogen (of which 28 % is water-insoluble)
and has an activity index of 50. Urea-formaldehyde
products are used to fertilize sod and certain speciality
crops. As a result of the slow nitrification pattern, ureaformaldehyde
prevents excessive leaching of nitrates.
The use of urea-formaldehyde is not popular because it is
costlier than the other nitrogenous fertilizers.
The condensation product of urea and acetaldehyde is
commercially known as urea-z. It is a slow-release
nitrogen fertilizer containing around 31 % nitrogen. Urea
crotonaldehyde, a derivative of urea (also known as
crotonylidene diurea), is also a slow-release nitrogen
fertilizer.
Urea-sulphur is a relatively new compound
containing 40% nitrogen and 10% sulphur. The prilled
material has excellent physical properties. Its urea part
dissolves after being applied to the soil, leaving elemental
sulphur that is converted into sulphate by the oxidizing
bacteria.
Adding urea slurry to diammonium phosphate slurry
(before or during granulation) makes urea-phosphate
which has a higher nitrogen-to-phosphorus ratio than the
ammonium phosphate does; the product contains 29%
nitrogenand 12.7% phosphorus (29.0% P2O5).
A new liquid fertilizer material [CO(NH2)2?H3PO4] is
made by the reaction of urea, phosphoric acid and water.
Depending on the ratio of the reactants, the nitrogen
content varies from 10% to 28% and the phosphorus
content from 9 to 18%. | [Trade name]
PRESPERSION, 75 UREA®; SUPERCEL
3000®; UREAPHIL®; UREOPHIL®; UREVERT®;
VARIOFORM II® | [Biochem/physiol Actions]
Urea solution is primarily used for protein denaturation. It also increases solubility of hydrocarbons and reduce micelle formation. Urea solution at high concentration leads to the destabilization of amyloid β16?22 oligomers. | [Environmental Fate]
Terrestrial Fate
Urea is expected to have very high mobility in soil. Urea is not
expected to volatilize from dry soil surfaces based on its vapor
pressure. Various field and laboratory studies have demonstrated
that urea degrades rapidly in most soils. Urea is rapidly hydrolyzed
to ammonium ions through soil urease activity, which
produces volatile gases, that is, ammonia and carbon dioxide.
However, the rate of hydrolysis can be much slower, depending
on the soil type, moisture content, and urea formulation.
Aquatic Fate
Urea is not expected to adsorb to suspended solids and sediments.
Volatilization from water surfaces is not expected. Urea
is rapidly hydrolyzed to ammonia and carbon dioxide in
environmental systems by the extracellular enzyme urease,
which originates from microorganisms and plant roots.
Atmospheric Fate
According to a model of gas/particle partitioning of semivolatile
organic compounds in the atmosphere, urea, which has
a vapor pressure of 1.2×10-5mm Hg at 251°C, will exist in
both the vapor and particulate phases in the ambient atmosphere.
Vapor-phase urea is degraded in the atmosphere by
reaction with photochemically produced hydroxyl radicals; the
half-life for this reaction in air is estimated to be 9.6 days. | [Metabolism]
The high analysis and good handling properties of urea
have made it the leading nitrogen fertilizer, both as
a source of nitrogen alone or when compounded with
other materials in mixed fertilizers. Although an excellent
source of nitrogen, urea can present problems unless
properly managed; due to its rapid hydrolysis to ammonia,
significant volatilization loss of this may occur if prilled
or granular urea is applied to and left on the soil
surface without timely incorporation. Mixtures of urea
and ammonium nitrate for use in mixed fertilizers are also
more highly hygroscopic than ammonium nitrate itself. | [storage]
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from oxidizers. Where possible, automatically transfer material fromstorage containers to process containers. | [Purification Methods]
Crystallise urea twice from conductivity water using centrifugal drainage and keeping the temperature below 60o. The crystals are dried under vacuum at 55o for 6hours. Levy and Margouls [J Am Chem Soc 84 1345 1962] prepared a 9M solution in conductivity water (keeping the temperature below 25o) and, after filtering through a medium-porosity glass sinter, added an equal volume of absolute EtOH. The mixture was set aside at -27o for 2-3 days and filtered cold. The precipitate was washed with a small amount of EtOH and dried in air. Crystallisation from 70% EtOH between 40o and -9o has also been used. Ionic impurities such as ammonium isocyanate have been removed by treating the concentrated aqueous solution at 50o with Amberlite MB-1 cation-and anion-exchange resin, and allowing it to crystallise on evaporation. [Benesch et al. J Biol Chem 216 663 1955.] It can also be crystallised from MeOH or EtOH, and is dried under vacuum at room temperature. [Beilstein 3 H 42, 3 I 19, 3 II 35, 3 III 80.] | [Toxicity evaluation]
The primary mechanism of toxicity appears to be inhibition of
the citric acid cycle. It leads to blockade of electron transport
and a decrease in energy production and cellular respiration,
which leads to convulsions. |
| Questions And Answer | Back Directory | [Chemical structure]
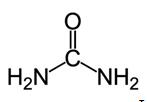 Lewis structure Lewis structure
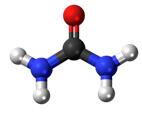 Ball-and-stick diagram Ball-and-stick diagram
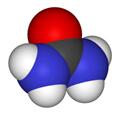 Space-filling model Space-filling model
Urea, also known as carbamide, is an organic compound with chemical formula CO (NH2)2. This amide has two –NH2 groups joined by a carbonyl (C=O) functional group. | [History]
Pure urea was first isolated from urine in 1727 by the Dutch scientist Herman Boerhaave, and he extracted urea from urine by working with the concentated-by-boiling residue. But if only not considering the purity of urea, the discovery of urea should be attributed to the French chemist Hilaire Rouelle, and he prepared urea (or its addition compound with sodium chloride) from urine some time before 1727.
In 1828, just 55 years after its discovery, urea became the first organic compound to be synthetically formulated, this time by a German chemist named Friedrich Wöhler, one of the pioneers of organic chemistry. It was found when Wohler attempted to synthesis ammonium cyanate, to continue a study of cyanates which he had been carrying out for several years. On treating silver cyanate with ammonium chloride solution he obtained a white crystalline material which proved identical to urea obtained from urine.
AgNCO + NH4Cl → (NH2)2CO + AgCl
Synthetic urea is created from synthetic ammonia and carbon dioxide and can be produced as a liquid or a solid. The process of dehydrating ammonium carbamate under conditions of high heat and pressure to produce urea was first implemented in 1870 and is still in use today. Uses of synthetic urea are numerous and therefore production is high. Approximately one million pounds of urea is manufactured in the United States alone each year, most of it used in fertilizers. Nitrogen in urea makes it water soluble, a highly desired property in this application.
| [Occurrence]
Urea is the chief nitrogenous end product of the metabolic breakdown of proteins in all mammals and some fishes. The material occurs not only in the urine of all mammals but also in their blood, bile, milk, and perspiration. In the course of the breakdown of proteins, amino groups (NH2) are removed from the amino acids that partly comprise proteins. These amino groups are converted to ammonia (NH3), which is toxic to the body and thus must be converted to urea by the liver. The urea then passes to the kidneys and is eventually excreted in the urine.
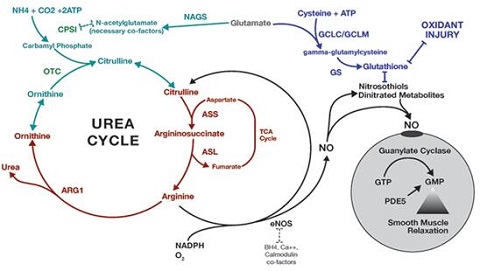
Fig.1 The urea cycle in animals | [Physical properties]

Fig.2 Urea crystal
It is a colourless, crystalline substance that melts at 132.7°C (271°F) and decomposes before boiling. Its density is 1.32 g/cm3 and It is highly soluble in water and contains 46.7% nitrogen. | [Chemical Properties]
The urea molecule is planar in the crystal structure, but the geometry around the nitrogens is pyramidal in the gas-phase minimum-energy structure. In solid urea, the oxygen center is engaged in two N-H-O hydrogen bonds. The resulting dense and energetically favourable hydrogen-bond network is probably established at the cost of efficient molecular packing: The structure is quite open, the ribbons forming tunnels with square cross-section. The carbon in urea is described as sp2 hybridized, the C-N bonds have significant double bond character, and the carbonyl oxygen is basic compared to, say, formaldehyde. Urea's high aqueous solubility reflects its ability to engage in extensive hydrogen bonding with water.
Urea dissolved in water is in equilibrium with the isomeric ammonium cyanate. The resulting activity of the isocyanic acid ions do result in carbamylation (formation of long-chain carbamides, liberating ammonia molecule as byproduct) of proteins if proteins are present in the solution too. The carbamylation reaction may occurs at elevated temperatures even without catalysts. At room temperature, water solutions of urea are prone to same decomposition reaction in the presence of urease. The isomerization of urea in solution at room temperature without catalysts is a slow process (taking days to reach equilibrium), and freshly prepared, unheated solutions had negligible carbamylation rates.Urea can react with alcohols to form urethanes and react with malonic esters to make barbituric acids.
| [Productions]
The primary raw material used to manufacture urea is natural gas, which ties the costs directly to gas prices. Consequently, new plants are only being built in areas with large natural gas reserves where prices are lower. Finished product is transported around the globe in large shipments of 30,000 metric tons. The market price for urea is directly related to the world price of natural gas and the demand for agricultural products. Prices can be very volatile, and at times, unpredictable. TCC is positioned to know the world markets and keep your prices competitive.
Annual production of sulfuric acid
Middle East
20 million tonnes
Rest of Asia
18 million tonnes
North America
9.5 million tonnes
Europe
9.5 million tonnes
1. Potash Corporation, 2013
2. International Fertilizer Industry Association, 2014
| [Production methods]
Historical process
Urea was first noticed by Hermann Boerhaave in the early 18th century from evaporates of urine. In 1773, Hilaire Rouelle obtained crystals containing urea from human urine by evaporating it and treating it with alcohol in successive filtrations. This method was aided by Carl Wilhelm Scheele's discovery that urine treated by concentrated nitric acid precipitated crystals. Antoine François, comte de Fourcroy and Louis Nicolas Vauquelin discovered in 1799 that the nitrated crystals were identical to Rouelle's substance and invented the term "urea." Berzelius made further improvements to its purificationand finally William Prout, in 1817, succeeded in obtaining and determining the chemical composition of the pure substance. In the evolved procedure, urea was precipitated as urea nitrate by adding strong nitric acid to urine. To purify the resulting crystals, they were dissolved in boiling water with charcoal and filtered. After cooling, pure crystals of urea nitrate form. To reconstitute the urea from the nitrate, the crystals are dissolved in warm water, and barium carbonate added. The water is then evaporated and anhydrous alcohol added to extract the urea. This solution is drained off and evaporated, leaving pure urea.
Industrial process
For use in industry, urea is produced from synthetic ammonia and carbon dioxide. As large quantities of carbon dioxide are produced during the ammonia manufacturing process as a byproduct from hydrocarbons (predominantly natural gas, less often petroleum derivatives), or occasionally from coal, urea production plants are almost always located adjacent to the site where the ammonia is manufactured.
Urea can be produced as prills, granules, pellets, crystals, and solutions. The prills are formed by spraying molten urea down a tower up which air is pumped. They are slightly smaller than urea sold as granules and are particularly useful when the fertilizer is being applied by hand. In admixture, the combined solubility of ammonium nitrate and urea is so much higher than that of either component alone that it is possible to obtain a stable solution (known as UAN) with a total nitrogen content (32%) approaching that of solid ammonium nitrate (33.5%), though not, of course, that of urea itself (46%).
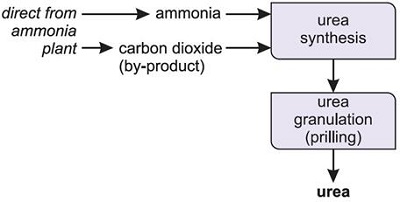
Fig.3 Industrial process of urea

Fig.4 An aerial view of a large plant in Alberta, Canada, in which ammonia is synthesized and then converted to urea.( By kind permission of Agrium Inc.)

Fig.5 Prills(small spheres of urea)

Fig.6 UAN(admixture of urea and ammonium nitrate)Laboratory process
Ureas in the more general sense can be accessed in the laboratory by reaction of phosgene with primary or secondary amines, proceeding through an isocyanate intermediate. Non-symmetric ureas can be accessed by reaction of primary or secondary amines with an isocyanate.
Also, urea is produced when phosgene reacts with ammonia:
COCl2 + 4 NH3 → (NH2)2CO + 2 NH4Cl
Urea is byproduct of converting alkyl halides to thiols via a S-alkylation of thiourea. Such reactions proceed via the intermediacy of isothiouronium salts:
RX + CS(NH2)2 → RSCX(NH2)2X
RSCX(NH2)2X + MOH → RSH + (NH2)2CO + MX
In this reaction R is alkyl group, X is halogen and M is alkali metal. | [Uses]
Agriculture uses
More than 90% of world industrial production of urea is destined for use as a nitrogen-release fertilizer. Urea has the highest nitrogen content of all solid nitrogenous fertilizers in common use. Therefore, it has the lowest transportation costs per unit of nitrogen nutrient.
In the soil, it hydrolyses back to ammonia and carbon dioxide. The ammonia is oxidized by bacteria in the soil to nitrate, which can be absorbed by the plants. Urea is also used in many multi-component solid fertilizer formulations. Urea is highly soluble in water, therefore, very suitable for use in fertilizer solutions (in combination with ammonium nitrate: UAN), e.g., in ‘foliar feed’ fertilizers. For fertilizer use, granules are preferred because of their narrower particle size distribution, an advantage for mechanical application. The most common impurity of synthetic urea, biuret, must be present at less than 2 percent of the time, as it impairs plant growth.
 
Fig.7 Urea fertilizer and farmer’s fertilization process
Pharmacaeutical
Urea and malonic acid react to form barbituric acid. This was discovered by Adolf Bayer in 1864. But the barbiturates were not exploited as hypnotics until the early 1900's.Urea is also used in the production of various acylureas and urethanes for use as sedatives and hypnotics.
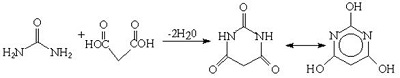
Fig.8 Synthesis of barbituric acid
Chemical industry
Urea is a raw material for the manufacture of two main classes of materials: urea-formaldehyde resins and urea-melamine-formaldehyde used in marine plywood. They all have very varied uses including adhesives, laminates, moulding compounds, coatings and textile finishes.
Urea has the ability to trap many organic compounds in the form of clathrates. The organic compounds are held in channels formed by interpenetrating helices comprising of hydrogen-bonded urea molecules. This behavior can be used to separate mixtures, and has been used in the production of aviation fuel and lubricating oils, and in the separation of paraffin.
As the helices are interconnected, all helices in a crystal must have the same molecular handedness. This is determined when the crystal is nucleated and can thus be forced by seeding. The resulting crystals have been used to separate racemic mixtures.
Laboratory uses
Urea in concentrations up to 10 M is a powerful protein denaturant as it disrupts the noncovalent bonds in the proteins. This property can be exploited to increase the solubility of some proteins. A mixture of urea and choline chloride is used as a deep eutectic solvent, a type of ionic liquid.
Urea can in principle serve as a hydrogen source for subsequent power generation in fuel cells. Urea present in urine/wastewater can be used directly (though bacteria normally quickly degrade urea.) Producing hydrogen by electrolysis of urea solution occurs at a lower voltage (0.37 V) and thus consumes less energy than the electrolysis of water (1.2 V).
Urea in concentrations up to 8 M can be used to make fixed brain tissue transparent to visible light while still preserving fluorescent signals from labeled cells. This allows for much deeper imaging of neuronal processes than previously obtainable using conventional one photon or two photon confocal microscopes.
Automobile systems
Urea is used in SNCR and SCR reactions to reduce the NOx pollutants in exhaust gases from combustion, for example, from power plants and diesel engines. The BlueTec system, for example, injects water-based urea solution into the exhaust system. The ammonia produced by decomposition of the urea reacts with the nitrogen oxide emissions and is converted into nitrogen and water within the catalytic converter.
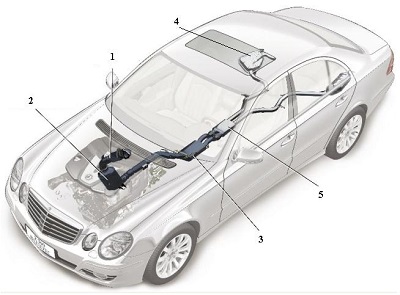
Fig.9 A line diagram of the car above illustrating five key elements in the design of the exhaust system.
1 The oxidation catalyst is used to remove unwanted hydrocarbons, ensuring that they are oxidised to carbon dioxide and water. The catalyst is usually based on platinum or palladium.
2 Known as an NOx catalytic convertor, it contains aluminium oxide on whose surface, platinum and barium oxide are present. It traps the oxides of nitrogen. When the solid is saturated with the oxides, unburnt hydrocarbons are allowed to flow through, converting much of the mixture to nitrogen, carbon dioxide and water vapour.
3 A filter which traps particulates (small pieces of carbon and other solids).
4 A tank containing the solution of urea.
5 The SCR-catalytic convertor which contains another catalyst, for example an oxide of vanadium (or tungsten) on titanium dioxide, which allows the exhaust gases, still containing some nitrogen oxides, to react with ammonia formed from the urea solution, to produce exhaust gases with only traces of the oxides. By kind permission of Daimler AG
Others
A stabilizer in nitrocellulose explosive
A component of animal feed, providing a relatively cheap source of nitrogen to promote growth
A non-corroding alternative to rock salt for road de-icing, and the resurfacing of snowboarding half pipes and terrain parks
A flavor-enhancing additive for cigarettes
A main ingredient in hair removers such as Nair or Veet
A browning agent in factory-produced pretzels
An ingredient in some hair conditioners, facial cleansers, bath oils, skin softeners, and lotions
A reactant in some ready-to-use cold compresses for first-aid use, due to the endothermic reaction it creates when mixed with water
A cloud seeding agent, along with other salts
A flame-proofing agent, commonly used in dry chemical fire extinguisher charges such as the urea-potassium bicarbonate mixture.
An ingredient in many tooth whitening products
An ingredient in dish soap
Along with ammonium phosphate, as a yeast nutrient, for fermentation of sugars into ethanol
A nutrient used by plankton in ocean nourishment experiments for geoengineering purposes
As an additive to extend the working temperature and open time of hide glue
As a solubility-enhancing and moisture-retaining additive to dye baths for textile dyeing or printing | [Hazards]
Health hazards
Inhalation:
Causes irritation to the respiratory tract. Symptoms may include coughing, shortness of breath. May be absorbed into the bloodstream with symptoms similar to ingestion.
Ingestion:
Causes irritation to the gastrointestinal tract. Symptoms may include nausea, vomiting and diarrhea. May also cause headache, confusion and electrolyte depletion.
Skin Contact:
Causes irritation to skin. Symptoms include redness, itching, and pain.
Eye Contact:
Causes irritation, redness, and pain.
Chronic Exposure:
A study of 67 workers in an environment with high airborne concentrations of urea found a high incidence of protein metabolism disturbances, moderate emphysema, and chronic weight loss.
Aggravation of Pre-existing Conditions:
Supersensitive individuals with skin or eye problems, kidney impairment or asthmatic condition should have physician's approval before exposure to urea dust.
Fire Hazards
Behavior in Fire: Melting and decomposing to generate ammonia.
Not combustible. Gives off irritating or toxic fumes (or gases) in a fire.
https://pubchem.ncbi.nlm.nih.gov/compound/urea#section=EPA-Safer-Chemical
Handling and Storage
Keep in a tightly closed container, stored in a cool, dry, ventilated area. Protect against physical damage. Isolate from incompatible substances. Containers of this material may be hazardous when empty since they retain product residues (dust, solids); observe all warnings and precautions listed for the product.
| [Reference]
- https://en.wikipedia.org/wiki/Urea#Explosives
- http://m.is0513.com/ProductChemicalPropertiesCB5853861_EN.htm
- https://chemistry.stackexchange.com/questions/54387/extracting-urea-from-urine/60338#60338
- http://www.chm.bris.ac.uk/motm/urea/urea.html
- https://thechemco.com/chemical/urea/
- file:///C:/Users/zl/Desktop/kurzer1956.pdf
- https://www.britannica.com/science/urea
- http://www.expertsmind.com/topic/biochemistry/urea-cycle-96120.aspx
- http://sesl.com.au/blog/what-is-urea/
- http://www.essentialchemicalindustry.org/chemicals/urea.html
- http://www.atmos.umd.edu/~russ/MSDS/urea.htm
|
|
|