| Identification | More | [Name]
3,5-Dibromobenzaldehyde | [CAS]
56990-02-4 | [Synonyms]
3,5-DIBROMOBENZALDEHYDE
3,5-Dibromobenzaldehyde97%MinByG.C
3,5-Dibromobenzaldehyde,98%
3,5-DIBROMOBENZALDEHYDE 98% | [EINECS(EC#)]
611-441-9 | [Molecular Formula]
C7H4Br2O | [MDL Number]
MFCD00156887 | [Molecular Weight]
263.91 | [MOL File]
56990-02-4.mol |
| Questions And Answer | Back Directory | [Description]
3,5-Dibromobenzaldehyde is a white or beige solid with a melting point
of 84-88 °C. Its boiling point and density are estimated to be
287.2±20.0 °C and 1.977±0.06 g/cm3, respectively. It is insoluble in water. | [Synthesis]
1,3,5-tribromobenzene (3.01 g, 9.6 mmol) in diethyl ether (80 mL) was
cooled to -78°C followed by the addition of one equivalent of n-BuLi
dropwise (2.5 M, 3.8 mL). The reaction was stirred for 30 minutes then
DMF (740 μL, 9.6 mmol) was added dropwise to the reaction and stirred at
-78°C for one hour. The vessel was then placed in an ice bath and
stirred for 30 minutes. A 10% HCl solution (100 mL) was added to quench
the reaction followed by CHCl3 (150 mL). The organic layer was collected and the aqueous layer washed with CHCl3 (80 mL). The organic layers where combined and dried over MgSO4
and the solvent removed. The crude product was purified by column
chromatography eluting with 10% EtOAc in hexanes. Spectral data for the
title compound was not reported in the literature reference. Yield: 1.93
g of the title compound (77%). 1H NMR (CDCl3, 300 MHz): δ = 9.90 (s, 1H), 7.92 (d, 2H), 7.60 (s, 1H); 13C NMR (CDCl3, 75 MHz) δ = 189.3, 139.7, 139.0, 131.37, 124.1; GC-MS [M+H]+ 262.8709, calcd 262.8707
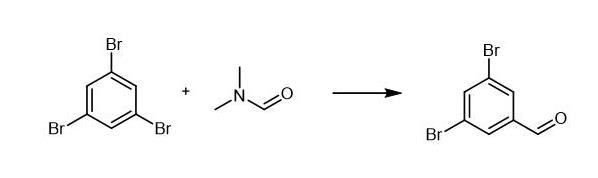 |
| Chemical Properties | Back Directory | [Appearance]
White powder | [Melting point ]
84-88 °C (lit.) | [Boiling point ]
287.2±20.0 °C(Predicted) | [density ]
1.977±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
soluble in DMSO, Ethyl Acetate, Methanol | [form ]
Powder or Crystals | [color ]
White to light beige | [Water Solubility ]
insoluble | [Sensitive ]
Air Sensitive | [BRN ]
2573432 | [InChI]
InChI=1S/C7H4Br2O/c8-6-1-5(4-10)2-7(9)3-6/h1-4H | [InChIKey]
ZLDMZIXUGCGKMB-UHFFFAOYSA-N | [SMILES]
C(=O)C1=CC(Br)=CC(Br)=C1 | [CAS DataBase Reference]
56990-02-4(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3261 8/PG 2
| [WGK Germany ]
3
| [HazardClass ]
8 | [PackingGroup ]
Ⅱ | [HS Code ]
29130000 |
| Hazard Information | Back Directory | [Chemical Properties]
White powder | [Uses]
3,5-Dibromobenzaldehyde is a dibrominated benzaldehyde that is a very useful building block for the preparation of a wide range of biologically active compounds such as a antibacterials | [Application]
Reactant involved in:
Suzuki-Miyaura cross-coupling reactions
Synthesis of blue fluorescent dye derivatives for organic light emitting diodes
Sharpless kinetic resolution for the formation of Baylis-Hillman enal adducts
Synthesis of podophyllotoxin mimetic pyridopyrazoles as anticancer agents
Allylic alkylation
Synthesis of C2-symmetric biphosphine ligand I | [References]
[1] Kuang, Yunsuo et al. “Novel AIEgens with a 3,5-dibromobenzaldehyde skeleton: molecular design, synthesis, tunable emission and detection application?.” Analytical Methods 46 (2018): 5486–5492.
|
|
|