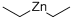| Identification | More | [Name]
Diethylzinc | [CAS]
557-20-0 | [Synonyms]
DIETHYLZINC
ZINCDIETHYL
Zinc ethide
ZINC ETHYL
(C2H5)2Zn
diethyl-zin
diethylzine
DIETHYLZINC, CYLINDER WITH 100 G
DIETHYLZINC SOLUTION, IN TOLUENE (1.1 M)
DIETHYLZINC, 15 WT. % (1.1M) SOLUTION IN TOLUENE
DIETHYLZINC SOLUTION, ~1 M SOLUTION IN H EXANE
DIETHYLZINC, 52.0 WT.% ZN (MINIMUM)
DIETHYLZINC, FOR CAMBRIDGE NANOTECH INC
DIETHYLZINC, 1.0M SOLUTION IN HEXANES
DIETHYLZINC, 1.0M SOLUTION IN HEPTANE
Diethylene Zinc
DEZn
Diethylzinc, 1.5M solution in toluene
Diethylzinc, 1M solution in hexane
Diethylzinc,elec.gr.(99.999%-Zn)PURATREM | [EINECS(EC#)]
209-161-3 | [Molecular Formula]
C4H10Zn | [MDL Number]
MFCD00009021 | [Molecular Weight]
123.51 | [MOL File]
557-20-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Slightly turbid light brown-grey solution | [Melting point ]
−28 °C(lit.)
| [Boiling point ]
98 °C
| [density ]
1.205 g/mL at 25 °C(lit.)
| [vapor pressure ]
16hPa at 20℃ | [refractive index ]
n20/D 1.498(lit.)
| [Fp ]
45 °F
| [storage temp. ]
0-6°C
| [solubility ]
reacts with H2O; msc ethyl ether,petroleum ether, benzene | [form ]
Solution | [color ]
Slightly turbid light brown-gray | [Specific Gravity]
0.740 | [Water Solubility ]
REACTS VIOLENTLY | [Hydrolytic Sensitivity]
8: reacts rapidly with moisture, water, protic solvents | [Sensitive ]
Air & Moisture Sensitive | [Merck ]
14,3131 | [BRN ]
3587207 | [Dielectric constant]
2.5(20℃) | [CAS DataBase Reference]
557-20-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Diethylzinc(557-20-0) | [EPA Substance Registry System]
557-20-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F,Xn,N,C | [Risk Statements ]
R14:Reacts violently with water.
R17:Spontaneously flammable in air.
R34:Causes burns.
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R67:Vapors may cause drowsiness and dizziness.
R65:Harmful: May cause lung damage if swallowed.
R62:Possible risk of impaired fertility.
R48/20:Harmful: danger of serious damage to health by prolonged exposure through inhalation .
R11:Highly Flammable.
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R14/15:Reacts violently with water, liberating extremely flammable gases .
R63:Possible risk of harm to the unborn child. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S62:If swallowed, do not induce vomiting: seek medical advice immediately and show this container or label .
S8:Keep container dry .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S16:Keep away from sources of ignition-No smoking .
S60:This material and/or its container must be disposed of as hazardous waste .
S43:In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add-Never use water) . | [RIDADR ]
UN 3399 4.3/PG 1
| [WGK Germany ]
2
| [RTECS ]
ZH2077777 | [F ]
10-23 | [TSCA ]
Yes | [HazardClass ]
4.3 | [PackingGroup ]
I | [HS Code ]
29319090 | [Safety Profile]
Presumed to be a
poison. Ignites spontaneously in air.
Dangerously flammable by spontaneous
chemical reaction in air, or with oxidzing
materials. A dangerous explosion hazard Explosive reaction with alkenes +
diodomethane, sulfur dioxide. Reacts
violently with bromine, water, nitro
compounds. Igmtes on contact with air,
ozone, methanol, or hydrazine. Reacts
violently with nonmetal halides (e.g., arsenic
trichloride or phosphorus trichloride) to
produce pyrophoric triethyl arsine or
triethyl phosphine. To fight fire, do not use
water, foam, or halogenated extinguishing
agents. Use dry materials, such as graphite,
sand, etc. When heated to decomposition it
emits toxic fumes of ZnO. See also ZINC
COMPOUNDS. | [Hazardous Substances Data]
557-20-0(Hazardous Substances Data) |
| Hazard Information | Back Directory | [General Description]
DIETHYLZINC(557-20-0) is a pyrophoric liquid with a garlic-like odor. DIETHYLZINC(557-20-0) is stable when DIETHYLZINC(557-20-0) is shipped in sealed tubes with carbon dioxide. DIETHYLZINC(557-20-0) may decompose violently in water and ignite spontaneously with air. DIETHYLZINC(557-20-0) is toxic by ingestion. If exposed to heat or flame, containers of this material may explode. DIETHYLZINC(557-20-0) is used as an aircraft fuel. | [Reactivity Profile]
DIETHYLZINC is pyrophoric in air, DIETHYLZINC ignites instantaneously. DIETHYLZINC reacts explosively with alcohols (methanol, ethanol), bromine, chlorine or liquefied sulfur dioxide [Houben-Weyl, 1973, 13.2a, p. 855, 757, 709]. Reaction with water, nitro compounds, arsenic trichloride, phosphorus trichloride is violent [Bretherick, 5th ed., 1995, p. 587]. | [Air & Water Reactions]
Highly flammable. Ignites in air with a blue flame giving off a peculiar garlic-like odor, [Merck, 11th ed., 1989]. Diethyl zinc is spontaneously flammable in air, [Douda(1966)]. Reacts violently with water to form flammable ethane gas, [Brauer(1965)]. | [Health Hazard]
Inhalation of mist or vapor causes immediate irritation of nose and throat; excessive or prolonged inhalation of fumes from ignition or decomposition may cause ``metal fume fever'' (sore throat, headache, fever, chills, nausea, vomiting, muscular aches, perspiration, constricting sensation in lungs, weakness, sometimes prostration); symptoms usually last 12-24 hrs., with complete recovery in 24-48 hrs. Eyes are immediately and severely irritated on contact with liquid, vapor, or dilute solution; without thorough irrigation, cornea may be permanently damaged. Moisture in skin combines with chemical to cause thermal and acid burns; tissue may be scarred without prompt treatment. Ingestion is unlikely but would cause immediate burns at site of contact; pain, nausea, vomiting, cramps, and diarrhea may follow; if untreated, tissue may become ulcerated. | [Description]
Diethyl zinc is an organometal compound and is a dangerous fire hazard. It spontaneously ignites in air and reacts violently with water, releasing flammable vapors and heat. It is a colorless, pyrophoric liquid with a specific gravity of 1.2, which is heavier than water, so it will sink to the bottom. It decomposes explosively at 248°F (120°C). It has a boiling point of 243°F (117°C), a flash point of ?20°F (?28°C), and a melting point of ?18°F (?27°C). The four-digit UN identification number is 1366. The NFPA 704 designation is health 3, flammability 4, and reactivity 3. The white space at the bottom of the diamond has a W with a slash through it to indicate water reactivity. Primary uses of diethyl zinc are in the polymerization of olefins, high-energy aircraft, and missile fuel and in the production of ethyl mercuric chloride. | [Chemical Properties]
Clear colorless solution | [Uses]
Diethyl zinc is used in organic synthesis. It isalso used in preservation of archival papers. | [Uses]
In organic synthesis; in preservation on archival papers. | [Definition]
ChEBI: Diethylzinc is a dialkylzinc compound. | [Fire Hazard]
Diethylzinc ignites spontaneously in air,
burning with a blue flame. Reactions with
water and lower alcohols can be violent.Violent reactions can occur with halogens,
halogenated hydrocarbons, nitroorganics,
oxidizers, sulfur dioxide, and chlorides of
phosphorus, arsenic, and antimony. With the
latter compounds, diethylzinc forms pyrophoric
triethylphosphine, triethyl arsine, and
triethylstibine, respectively. | [Flammability and Explosibility]
Pyrophoric | [Synthesis]
Diethylzinc is obtained by the reaction of Zn and C2H5Ⅰ to produce C2H5ZnⅠ, which is then distilled under inert gas conditions.
Zn + C2H5Ⅰ = C2H5ZnⅠ; 2C2H5ZnⅠ = Zn(C2H5)2+ ZnⅠ2. | [Potential Exposure]
Diethyl zinc is used in organic syntheses; as a catalyst in the manufacture of olefin polymers andas a high-energy aircraft and missile fuel. | [First aid]
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. | [storage]
Color Code—Red Stripe: Flammability Hazard:Store separately from all other flammable materials. Priorto working with diethyl zinc you should be trained on itsproper handling and storage. Before entering confined spacewhere diethyl zinc may be present, check to make sure thatan explosive concentration does not exist. Diethyl zinc mustbe stored to avoid contact with water, chlorine, hydrazine,and oxidizers since violent reactions occur. Store in sealedtubes or cylinders under a dry, inert gas blanket or in anevacuated system. Shade from radiant heat and protect fromrain. Metal containers involving the transfer of=gallons ormore of diethyl zinc should be grounded and bonded.Wherever diethyl zinc is used, handled, manufactured, orstored, use explosion proof electrical equipment and fittings. Use only nonsparking tools and equipment, especiallywhen opening and closing containers of this chemical.Sources of ignition, such as smoking and open flames, areprohibited where this chemical is used, handled, or stored ina manner that could create a potential fire or explosionhazard. | [Shipping]
This compound requires a shipping label of“SPONTANEOUSLY COMBUSTIBLE.” It falls in Hazard4.2 and Packing Group I. | [Incompatibilities]
Ignites spontaneously on contact with airor strong oxidizers. Explosive decomposition at 245°F/120℃. Violent reaction with hydrazine, sulfur dioxide,halogens, some alcohols, ozone; possible fire and explosions. Contact with water forms ethane gas. |
|
|