| Identification | More | [Name]
Methazolamide | [CAS]
554-57-4 | [Synonyms]
METHAZOLAMIDE
N-[4-METHYL-2-SULFAMOYL-DELTA2-1,3,4-THIADIAZOLIN-5-YLIDENE] ACETAMIDE
2-acetylimino-3-methyl-delta(sup4)-1,3,4-thiadiazoline-5-sulfonamide
2-Acetylimino-3-methyl-delta4-1,3,4-thiadiazoline-5-sulfonamide
5-acetylimino-4-methyl-delta(sup2)-1,3,4-thiadiazoline-2-sulfonamide
5-Acetylimino-4-methyl-delta2-1,3,4-thiadiazoline-2-sulfonamide
Acetamide, N-(4-methyl-2-sulfamoyl-delta2-1,3,4-thiadiazolin-5-ylidene)-
Acetamide, N-[5-(aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-ylidene]-
Methenamide
N-((2Z)-5-(Aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-ylidene)acetamide
n-(4-methyl-2-sulfamoyl-delta(2)-1,3,4-thiadiazolin-5-ylidene)-acetamid
n-(4-methyl-2-sulfamoyl-delta(sup2)-1,3,4-thiadiazolin-5-ylidene)-acetamid
N-(4-Methyl-2-sulfamoyl-delta2-1,3,4-thiadiazolin-5-ylidene)acetamide
n-(5-(aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3h)-ylidene)-acetamid
Naptazane
Neptazane
Neptazaneat
N-(3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-2-ylidene)-acetamide
n-(4-methyl-2-sulfamoyl-△2-1,3,4-thiadiazolin-5-ylidene) acetamide
N-[(2E)-5-(Aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-ylidene]acetamide | [EINECS(EC#)]
209-066-7 | [Molecular Formula]
C5H8N4O3S2 | [MDL Number]
MFCD00083416 | [Molecular Weight]
236.27 | [MOL File]
554-57-4.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S22:Do not breathe dust .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
AC6350000
| [HS Code ]
2935904000 | [Hazardous Substances Data]
554-57-4(Hazardous Substances Data) |
| Hazard Information | Back Directory | [Description]
Methazolamide is a carbonic anhydrase inhibitor (IC50 = 130 nM).1 It reduces intraocular pressure and cerebrospinal fluid flow in a rat model of glaucoma. Methazolamide reduces electroshock-induced seizures in rats with an ED50 value of 19.2 mg/kg.2 It also inhibits production of reactive oxygen species (ROS) in a primary cortical neuron (PCN) cellular model of subarachnoid hemorrhage (SAH) and reduces cerebral edema in a mouse model of SAH.3 Methazolamide is larvicidal, with a larvicidal concentration (LC50) value of 724 ppm, but has no activity when administered in the diet to adult A. aegypti.4 Formulations containing methazolamide have been used for the treatment of glaucoma. | [Chemical Properties]
White Solid | [Originator]
Neptazane ,Lederle,US,1959 | [Uses]
Action of this drug is similar to that of acetazolamide, and it is used for lowering intraocular
pressure in treating wide-angle and secondary glaucoma, and before surgical intervention
for severe wide-angle glaucoma. | [Uses]
CNS & respiratory stimulant | [Uses]
Methazolamide is a carbonic anhydrase inhibitor. Methazolamide is used in the treatment of glaucoma. | [Definition]
ChEBI: Methazolamide is a member of thiadiazoles and a sulfonamide. | [Manufacturing Process]
A suspension of 6 parts by weight of 5-acetylimino-4-methyl-2- benzylmercapto-δ2-1,3,4-thiadiazoline in 180 parts by volume of 33% aqueous acetic acid was chlorinated at 5°C for 30 minutes. The solid was filtered off, dried, and added portion-wise to 100 parts by volume of liquid ammonia. The ammonia was removed under a stream of dry nitrogen.
The residual solid was partially dissolved in 10 parts by volume of water, filtered, and acidified to give 5-acetylimino-4-methyl-δ2-1,3,4-thiadiazoline-2- sulfonamide. The product was purified by two recrystallizations from hot water. | [Therapeutic Function]
Carbonic anhydrase inhibitor | [Biochem/physiol Actions]
Methazolamide is a cell-permeable and potent carbonic anhydrase (CA) inhibitor that is used in the treatment of glaucoma. Methazolamide is an insulin sensitizer that reduces hepatic glucose generation in animal models. | [Clinical Use]
Methazolamide is a derivative of acetazolamide in which one of the active hydrogens has been replaced by a methyl group. This decreases the polarity and permits a
greater penetration into the ocular fluid, where it acts as a carbonic anhydrase inhibitor, reducing intraocular pressure. Its dose for glaucoma is 50 to 100 mg two to three
times a day. | [Synthesis]
Methazolamide, N-(4-methyl-2-sulfamoyl-1,3,4-thiadiazol-5-yliden)
acetamide (21.2.3), is made by an intermediate product of acetazolamide synthesis?a
2-acetylamino-5-mercapto-1,3,4-thadiazol (9.7.3). This is benzylated with benzylchloride at
the mercapto group, forming 2-acetylamino-5-benzylthio-1,3,4-thiadiazole (21.2.1). Further
methylation of the product with methyl iodide leads to the formation of N-(4-methyl-
2-benzylthio-1,3,4-thiadiazol-5-yliden)acetamide (21.2.2). Oxidation and simultaneous
chlorination of the resulting product with chlorine in an aqueous solution of acetic acid, and
reacting the resulting chlorosulfonic derivative with ammonia gives (21.2.3).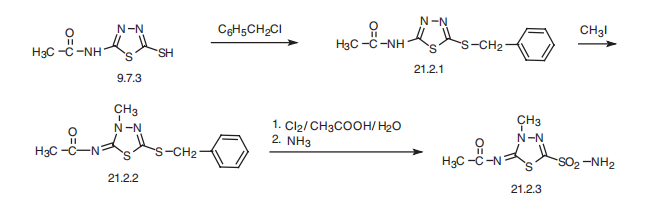
| [Veterinary Drugs and Treatments]
Orally administered methazolamide is used for the medical treatment
of glaucoma. |
|
|