| Identification | More | [Name]
Hydroxylamine hydrochloride | [CAS]
5470-11-1 | [Synonyms]
HA HCL
HOHCL
HYDROXYAMMONIUM CHLORIDE
HYDROXYLAMINE HCL
HYDROXYLAMINE/HCL SOLUTION
HYDROXYLAMINE HYDROCHLORIDE
HYDROXYLAMMONIUM CHLORIDE
OXAMMONIUM HCL
OXAMMONIUM HYDROCHLORIDE
hydroxyaminehydrochloride
hydroxylaminechloride
hydroxylaminechloride(1:1)
Oxammionium
HYDROXYLAMINE HYDROCHLORID
HYDROXYLAMINE HYDROCHLORIDE, FOR AAS
Hydroxylamine hydrochloride, 99.999% metals basis
HYDROXYLAMINE HYDROCHLORIDE REAGENTPLU&
HYDROXYLAMINE HYDROCHLORIDE, 99%, A.C.S. REAGENT
Hydroxylamine hydrochloride, 99.9999% metals basis
HYDROXYLAMINE HYDROCHLORIDE ACS REAGENT | [EINECS(EC#)]
226-798-2 | [Molecular Formula]
ClH4NO | [MDL Number]
MFCD00051089 | [Molecular Weight]
69.49 | [MOL File]
5470-11-1.mol |
| Chemical Properties | Back Directory | [Appearance]
White crystals | [Melting point ]
155-157 °C (dec.)(lit.)
| [bulk density]
900kg/m3 | [density ]
1.67 g/mL at 25 °C(lit.)
| [vapor pressure ]
0.054 Pa (50 °C) | [storage temp. ]
Store at RT. | [solubility ]
470g/l | [form ]
Liquid | [color ]
White to off-white | [PH]
2.5-3.5 (25℃, 50mg/mL in H2O) | [Stability:]
Substances to be avoided include strong oxidizing agents. Heating above 115 C may cause explosion; do not store above 65C. Moisture and air sensitive. | [Water Solubility ]
560 g/L (20 ºC) | [Sensitive ]
Hygroscopic | [Merck ]
14,4828 | [BRN ]
3539763 | [Contact allergens]
Hydroxylamine and its salts are used in various
branches of industry, as reducing agents in color film
developers or as reagents in laboratories. | [InChIKey]
WTDHULULXKLSOZ-UHFFFAOYSA-N | [LogP]
-0.810 (est) | [Uses]
Organic synthesis, photographic developer,
medicine, controlled reduction reactions, nondiscoloring short-stopper for synthetic rubbers, antioxidant for fatty acids. | [CAS DataBase Reference]
5470-11-1(CAS DataBase Reference) | [EPA Substance Registry System]
5470-11-1(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
White crystals | [General Description]
Colorless or off-white crystalline solid. pH (0.1 molar aqueous solution) 3.4. pH (0.2 molar aqueous solution) 3.2. | [Reactivity Profile]
A powerful reducing agent. Reacts with bases and oxidizing agents. | [Air & Water Reactions]
Hygroscopic. Sensitive to prolonged exposure to air. Water soluble. Reacts slowly with water. | [Hazard]
Toxic by ingestion, strong irritant to tissue. | [Fire Hazard]
Flash point data for this chemical are not available; however, HYDROXYLAMINE HYDROCHLORIDE is probably combustible. | [Description]
Hydroxylamine hydrochloride is a reducing agent that is routinely used for the deacetylation of SATA to form free sulfhydryls (Figure 1), for cleavage of protein cross‐linkers that contain carbonyl groups (i.e. EGS) and for mutagenesis of plasmid DNA.
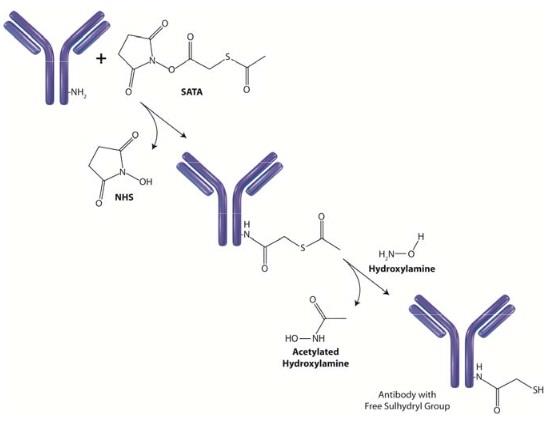
Hydroxylamine converts aldehydes and ketones (carbonyls) to their oxime derivative in weak bases, therefore cross‐linkers and other compounds with carbonyl groups are cleavable with Hydroxylamine hydrochloride.
SATA and SATP are modification reagents that add a sulfhydryl group to primary amines on biomolecules. The initial modification results in the addition of an acetyl‐protected sulfur enabling storage of the biomolecule. To generate a free sulfhydryl the biomolecule is treated with hydroxylamine to remove the protecting acetyl group (see figure).
EGS and sulfo‐EGS are homobifunctional, succinimidyl ester, amine reactive crosslinkers that are resistant to cleavage by denaturants used in SDS‐PAGE conditions, but may be cleaved with hydroxylamine. | [Physical properties]
Colorless monoclinic crystals; hygroscopic; decomposes slowly in moist air;density 1.67 g/cm3at 17°C; melts at 151°C (decomposes); highly soluble inwater (84g/100g at 20°C); soluble in lower alcohols and glycols; pH of 0.1molar solution 3.4. | [Characteristics]
Features of Hydroxylamine hydrochloride:
Quench amine-labeling or crosslinking reactions (e.g., with NHS esters)
Expose protected sulfhydryl groups on SATA-modified molecules
Cleave carbonyl-containing crosslinkers such as EGS and Sulfo-EGS
| [Definition]
ChEBI: Hydroxylamine hydrochloride is an organic molecular entity. It is a colorless inorganic compound (HONH2) used in organic synthesis and as a reducing agent, due to its ability to donate nitric oxide. | [Preparation]
Hydroxylamine hydrochloride is prepared by electrolytic reduction ofammonium chloride. | [Biotechnological Applications]
Hydroxylamine hydrochloride(5470-11-1) is a strong reducing agent that is useful in biochemical crosslinking applications, including the deacetylation of SATA and chemical cleavage of EGS and Sulfo-EGS. Hydroxylamine converts carbonyl compounds (aldehydes and ketones) to their oxime derivatives in the presence of a weak base. Therefore, crosslinkers and other compounds that contain a carbonyl within their structure are cleavable with hydroxylamine?HCl.
EGS and its water-soluble analog, Sulfo-EGS, are homobifunctional, succinimidyl ester, amine-reactive crosslinkers useful for covalent stabilization of polypeptide multimers and protein:protein interactions. Unlike disulfidecontaining crosslinkers, EGS and Sulfo-EGS will not cleave by reducing SDS-PAGE conditions but may be cleaved when necessary with hydroxylamine.
SATA and SATP are modification reagents for addition of sulfhydryl groups to proteins and other molecules containing primary amines. Such sulfhydryl addition is an important step in one popular method for preparing protein conjugates such as antibodies with horseradish peroxidase enzyme. The initial modification results in addition of an acetyl-protected sulfur, enabling storage of the modified protein; to make the sulfur available as a sulhydryl group (-SH) for the final conjugation reaction, hydroxylamine is used to remove the protecting acetyl group.
Hydroxylamine?HCl is more stable to oxidation than the free base form of hydroxylamine; nevertheless, always prepare hydroxylamine solutions immediately before use and store the product desiccated. Hydroxylamine?HCl is soluble in polar solvents such as water, ethanol, methanol, glycerol and propylene glycol.
| [Flammability and Explosibility]
Notclassified | [reaction suitability]
reaction type: C-H Activation
reagent type: catalyst | [Biochem/physiol Actions]
MAO inhibitor; inhibits platelet aggregation. | [Purification Methods]
Crystallise the salt from aqueous75% ethanol or boiling methanol, and dry it under vacuum over CaSO4 or P2O5. It has also been dissolved in a minimum of water and saturated with HCl; after three such crystallisations, it is dried under a vacuum over CaCl2 and NaOH. Its solubility at 20o is 85% in H2O, 6% in EtOH and 12% in MeOH. [Hurd Inorg Synth I 87 1939, Semon in Org Synth Coll Vol I 318 1941.] |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,N | [Risk Statements ]
R22:Harmful if swallowed.
R36/38:Irritating to eyes and skin .
R43:May cause sensitization by skin contact.
R48/22:Harmful: danger of serious damage to health by prolonged exposure if swallowed .
R50:Very Toxic to aquatic organisms. | [Safety Statements ]
S22:Do not breathe dust .
S24:Avoid contact with skin .
S37:Wear suitable gloves .
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [RIDADR ]
UN 2923 8/PG 2
| [WGK Germany ]
3
| [RTECS ]
NC3675000
| [F ]
21 | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
28251000 | [Toxicity]
LD50 orally in mice: 408 mg/kg (Riemann) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Sodium nitrite-->Dimethyl sulfate-->Water-->Sodium metabisulfite-->Nitromethane-->Acetone oxime | [Preparation Products]
2-AMINO-5,6-DICHLOROBENZOIC ACID-->ETHYL 5-METHYLISOXAZOLE-3-CARBOXYLATE-->5-METHYL-4-ISOXAZOLESULFONYL CHLORIDE-->5-AMINOISOXAZOLE-4-CARBOXYLIC ACID ETHYL ESTER-->4-Aminotetrahydropyran hydrochloride-->6-Bromoisatin-->3,4,5-Trimethoxybenzylamine-->Methyl 3-amino-4-methylthiophene-2-carboxylate-->4-AMINO-1,2,5-OXADIAZOLE-3-CARBONITRILE-->3-(2,6-Dichlorophenyl)-5-methylisoxazole-4-carboxylic acid-->ETHYL 3-(2,6-DICHLORO-PHENYL)-5-METHYL-ISOXAZOLE-4-CARBOXYLATE-->Epiandrosterone acetate-->6-AMINO-2-METHYL-2-HEPTANOL-->5-Methyl-3-phenylisoxazole-->2,6-Dicyano-4-Nitroaniline-->(2,6-DIMETHYLPHENOXY)ACETOXIME-->2-Butanone oxime-->3-METHYL-5-PHENYL-4-ISOXAZOLECARBOXYLIC ACID-->4-(METHYLTHIO)BENZYLAMINE-->heptanal oxime-->3-(2-Chlorophenyl)-5-methylisoxazole-4-carbonyl chloride-->BENZAMIDOXIME HYDROCHLORIDE-->2-Pyridylamid oxime-->NEMONAPRIDE-->3-(2-Chlorophenyl)-5-methylisoxazole-4-carboxylic acid-->Adrafinil-->17-Ethinylandrost-5-ene-3,17-diol-->5-Methyl-3-phenylisoxazole-4-carbonyl chloride-->4-Pyridylamidoxime-->3-PYRIDYLAMIDOXIME-->3-METHYL-5-PHENYLISOXAZOLE-->3-METHYLTHIOPHENE-2-CARBONITRILE-->N-Hydroxysulfosuccinimide sodium salt-->DIPHENYLGLYOXIME-->5-Chloro-3,6-dihydroxy-5-androstan-17-one 3-acetate-->2,3-DIMETHOXYBENZONITRILE-->5 A-CHLORO-6 B,19-EPOXY-3 B -HYDROXY-5 A-ANDROSTAN-17-ONE-->2-CHLOROBENZALOXIME-->Pyrazole-1,3-dimethyl-5-phenoxy-4-carboxaldehyde oxime-->Ethyl 5-methyl-3-phenylisoxazole-4-carboxylate |
|
|