| Identification | More | [Name]
(+)-5-Iodo-2'-deoxyuridine | [CAS]
54-42-2 | [Synonyms]
1-(2-DEOXY-BETA-D-RIBOFURANOSYL)-5-IODOURACIL
2'-DEOXY-5-IODOURIDINE
5-IODO-2'-DEOXY-D-URIDINE
(+)-5-IODO-2'-DEOXYURIDINE
(+)-5-IODO-2-DEOXYURIDINE
5-IODO-2'-DEOXYURIDINE
5-IODO-2-DEOXYURIDINE
5-IODODEOXYURIDINE
5-IODODESOXYURIDINE
DENDRID
EMANIL
IDOXURIDINE
IDU
IUDR
1-beta-d-2’-deoxyribofuranosyl-5-iodouracil
1beta-D-2'-Deoxyribofuranosyl-5-iodouracil
2’-deoxy-5-iodo-uridin
5-Iodouracil deoxyriboside
5-iodouracildeoxyriboside
5iudr | [EINECS(EC#)]
200-207-8 | [Molecular Formula]
C9H11IN2O5 | [MDL Number]
MFCD00134656 | [Molecular Weight]
354.1 | [MOL File]
54-42-2.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline Solid | [Melting point ]
194 °C (lit.) | [alpha ]
280 º (c=1,1M NaOH) | [density ]
1.7911 (estimate) | [refractive index ]
30 ° (C=1, 1mol/L NaOH) | [storage temp. ]
2-8°C
| [solubility ]
DMSO (Slightly, Sonicated), Methanol (Slightly, Heated, Sonicated) | [form ]
Crystalline Powder | [pka]
8.25(at 25℃) | [color ]
White to slightly beige | [biological source]
synthetic (organic) | [Water Solubility ]
1.6 g/L (20 ºC) | [Sensitive ]
Air & Light Sensitive | [Usage]
Used as an antiviral | [Detection Methods]
T,NMR,HPLC,Rotation | [Merck ]
14,4891 | [BRN ]
30397 | [InChIKey]
XQFRJNBWHJMXHO-FSDSQADBSA-N | [CAS DataBase Reference]
54-42-2(CAS DataBase Reference) | [NIST Chemistry Reference]
Uridine, 2'-deoxy-5-iodo-(54-42-2) | [Storage Precautions]
Light sensitive | [EPA Substance Registry System]
54-42-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T,Xn | [Risk Statements ]
R45:May cause cancer.
R61:May cause harm to the unborn child.
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36:Wear suitable protective clothing .
S22:Do not breathe dust . | [WGK Germany ]
3
| [RTECS ]
YU7700000
| [F ]
8-23 | [TSCA ]
Yes | [HS Code ]
29389090 | [Safety Profile]
Moderately toxic by
intraperitoneal route. Experimental
teratogenic and reproductive effects.
Questionable carcinogen with experimental
carcinogenic data. Human mutation data
reported. When heated to decomposition it
emits very toxic fumes of Iand NOx. | [Hazardous Substances Data]
54-42-2(Hazardous Substances Data) | [Toxicity]
LD50 i.p. in mice: 2.5 g/kg (Prusoff, 1979) |
| Hazard Information | Back Directory | [Description]
5-Iodo-2'-deoxyuridine is a nucleoside analog that inhibits the replication of viruses and other DNA-containing organisms. 2'-Deoxy-5-iodouridine also has inhibitory properties on cell nuclei, which may be due to its ability to bind with DNA and prevent the synthesis of RNA or protein. | [Chemical Properties]
Crystalline Solid | [Originator]
Dendrid,Alcon,US,1963 | [Uses]
A cytotoxic analog of thymidine, antiviral | [Uses]
5-Iodo-2'-deoxyuridine is antitumor nucleoside enantiomer thymidine kinase used as potential antiviral agents.
| [Uses]
Antiviral;Nucleic acid synthesis inhibitors | [Uses]
Idoxuridine is an antiviral agent effective against herpes-simplex infections; in ophthalmie eyedrops, ointments, and solutions. | [Uses]
Used as an antiviral | [Definition]
ChEBI: A pyrimidine 2'-deoxyribonucleoside compound having 5-iodouracil as the nucleobase; used as an antiviral agent. | [Indications]
Idoxuridine (Herplex) is a water-soluble iodinated derivative
of deoxyuridine that inhibits several DNA viruses
including HSV, VZV, vaccinia, and polyoma virus. The
triphosphorylated metabolite of idoxuridine inhibits
both viral and cellular DNA synthesis and is also incorporated
into DNA. Such modified DNA is susceptible to
strand breakage and causes aberrant viral protein synthesis.
Because of its significant host cytotoxicity, idoxuridine
cannot be used to treat systemic viral infections. The
development of resistance to this drug is common. | [Manufacturing Process]
5 g of 5-iodo-uracil (obtained according to T.B. Johnson et al., J. Biol. Chem.
1905/6, 1, 310) in 15 cc of acetic anhydride are heated under reflux for 4,5
hours. The acetylated derivative crystallizes on cooling. The crystallized
product is chilled for ? hour then filtered with suction, washed with acetic
anhydride and then with ether and dried. 4.5 g of 1-acetyl-5-iodo-uracil, MP
167°C, are thus obtained.
1.51 g of mercuric acetate are dissolved in 50 cc of methanol under reflux and
1.35 g of 1-acetyl-5-iodo-uracilare added. A white precipitate is soon formed.
The reaction mixture is kept under reflux for % hour and then allowed to cool to room temperature. The precipitate is then filtered with suction, washed
with methanol and dried.
2.1 g of monomercuric 5-iodo-uracil, MP 280°C, are thus obtained as a
colorless powder, insoluble in water and the majority of the usual organic
solvents, such as benzene, chloroform, alcohol, ether and acetone.
1.46 g of 5-iodo-uracil monomercuric derivative are introduced into 50 cc of
chloroform and 20 to 30 cc of the solvent are distilled off under normal
pressure to ensure good dehydration of the reaction medium. The mixture is
cooled to room temperature and 2.59 g of 3,5-di-p-toluyl-desoxy-D�ribofuranosyl chloride added. The mixture is agitated for 6 hours with glass
balls, filtered, rinsed with chloroform and the filtrate is successively washed
with an aqueous sodium iodide solution, with water, with a saturated solution
of sodium bicarbonate and again with water. The product is dried over sodium
sulfate, filtered and evaporated to dryness.
The residue crystallizes in ether and yields about 600 mg of β-3',5'-di-p�toluyl-2'-desoxy-5-iodo-uridine which is recrystallized from toluene. The
product is obtained as colorless crystals, soluble in chloroform and pyridine,
sparingly soluble in acetone, benzene ether and alcohol, insoluble in water, MP
193°C.
206 mg of 3',5'-di-p-toluyl-2'-desoxy-5-iodo-uridineare heated at 80°C with
2.5 cc of caustic soda solution (0.4 N) for ? hour. The solution obtained is
cooled, filtered and then acidified with acetic acid. The desoxy-iodo-uridine
and the p-toluic acid crystallize. Ether is added to dissolve the p-toluic acid,
the mixture is chilled, filtered with suction, washed with water and ether, and
dried. The residue is recrystallized from water and 100 mg of 5-iodo-2'-
desoxy-uridine, are obtained. | [Brand name]
Dendrid (Alcon);
Herplex (Allergan); Stoxil (GlaxoSmithKline). | [Therapeutic Function]
Antiviral (ophthalmic) | [Pharmaceutical Applications]
A halogenated pyrimidine analog originally synthesized as an
anticancer agent. Formulated in dimethylsulfoxide for topical
application and as a solution for ophthalmic use.
Activity is largely limited to DNA viruses, primarily HSV-1,
HSV-2 and VZV. HSV-1 plaque formation in BHK 21 cells
is sensitive to 6.25–25 mg/L; type 2 microplaques required
62.5–125 mg/L. RNA viruses are not affected, with the exception
of oncogenic RNA viruses such as Rous sarcoma virus.
Drug resistance is easily generated in vitro, and may be an
obstacle to treatment. However, there is little or no crossresistance
with newer nucleoside analogs.
It is poorly soluble in water, and aqueous solutions are
ineffective against infections other than those localized to the
eye. In animals, therapeutic levels are achieved in the cornea
within 30 min of ophthalmic application and persist for 4 h.
Penetration is otherwise poor, with only the biologically inactive
dehalogenated metabolite uracil entering the eye.
The drug is too toxic for systemic administration. Contact
dermatitis, punctate epithelial keratopathy, follicular conjunctivitis,
ptosis, stenosis and occlusion of the puncta and keratinization
of the lid margins occur in up to 14% of those
receiving ophthalmic preparations.
It is used in herpes keratitis, but has largely been superseded
by trifluridine or aciclovir. | [Biochem/physiol Actions]
5-Iodo-2′-deoxyuridine prevents in vitro DNA viral replication. This is observed in herpesviruses and poxviruses It might possess teratogenic, tumor-promoting, mutagenic, and immunosuppressive properties. 5-Iodo-2′-deoxyuridine, used in topical applications, is effective against epithelial infections. | [Mechanism of action]
Idoxuridine is a nucleoside containing a halogenated pyrimidine and is an analogue of thymidine. It
acts as an antiviral agent against DNA viruses by interfering with their replication based on the similarity
of structure between thymidine and idoxuridine. Idoxuridine is first phosphorylated by the host cell virus�encoded enzyme thymidine kinase to an active triphosphate form. The phosphorylated drug inhibits
cellular DNA polymerase to a lesser extent than HSV DNA polymerase, which is necessary for the
synthesis of viral DNA. The triphosphate form of the drug is then incorporated during viral nucleic acid
synthesis by a false pairing system that replaces thymidine. When transcription occurs, faulty viral
proteins are formed, resulting in defective viral particles. | [Clinical Use]
The only FDA-approved use of idoxuridine is in the
treatment of herpes simplex infections of the eyelid, conjunctiva conjunctiva,
and cornea. It is most effective against surface
infections because it has little ability to penetrate the tissues
of the eye. intravenous idoxuridine was designated
an orphan drug for the treatment of soft tissue sarcoma. | [Side effects]
Idoxuridine may cause local irritation, mild edema, itching,
and photophobia. Corneal clouding and small punctate
defects in the corneal epithelium have been reported.
Allergic reactions are rare. | [Synthesis]
Idoxuridine, 5-iodo-1-(2-deoxyyribofuranosyl)pyrimidin-2,4-(1H.3H)-dione
(36.1.14), is synthesized by the following scheme. 5-Iodouracil is acylated with acetic anhy�dride to make 1-acetyl-5-iodouracil (36.1.11). Treating this with mercury(II) acetate gives 5-iodomonomercury uracil (36.1.12), which is reacted with 1-bromodidesoxy-D-ribofura�nosyl-3,5-bis-(p-toluenesulfonate) to make a ditosyl derivative (36.1.13). Hydrolysis of the
tosyl groups using sodium hydroxide and subsequent treatment of the resulting substance
with acetic acid gives the desired product idoxuridine.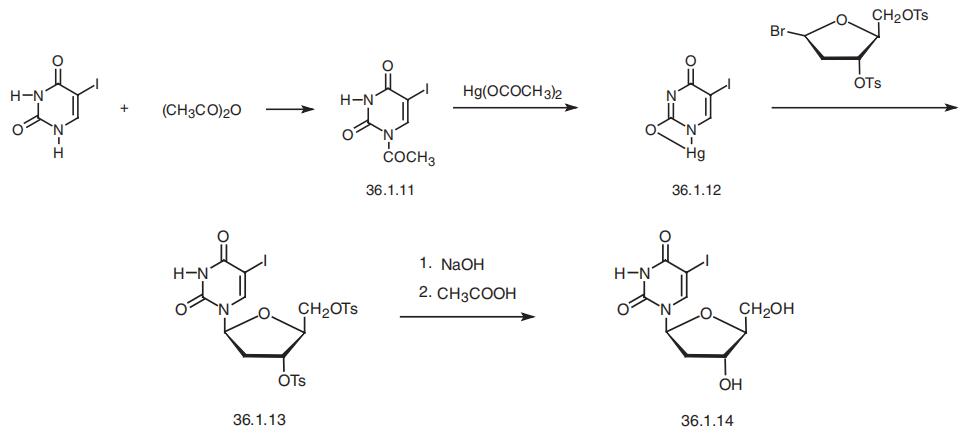
| [Veterinary Drugs and Treatments]
Idoxuridine (IDU) is chemically similar to thymidine and its substitution
into viral DNA causes misreading of the viral genetic code
thereby inhibiting viral replication. Like trifluridine, IDU is considered
virostatic rather than viricidal. IDU was found to be second
to trifluridine in efficacy in vitro against common strains of feline
herpes virus growing in kidney epithelial cells. IDU is extremely
well tolerated in cats and this feature alone makes it the most popular
antiviral currently available for use in cats with presumed or
established feline herpes virus infection. Although trifluridine was
shown to be more effective in vitro, the topical irritation it induces
in cats frequently negates any beneficial effect that might be noted
clinically. Stinging upon application is a rare feature with IDU/artificial
tear preparations. | [Metabolism]
Idoxuridine is metabolized rapidly in the body to iodouracil, uracil, and iodide. Metabolites are excreted in the urine.
|
|
|