| Identification | More | [Name]
Guanidine nitrate | [CAS]
506-93-4 | [Synonyms]
AMINOMETHANAMIDINE NITRATE
Guanidine mononitrate
GUANIDINE NITRATE
GUANIDINIUM NITRATE
GUN
Carbamidinenitrate
guanidinenitrate(1:1)
Iminoureamitrate
Uraminenitrate
GUANIDINIUM NITRATE 98+%
GuanidineNitratePure
Guanidinnitrat
GUANIDINENITRATE
AMINOGUANIDINE DINITRATE
Guanidine Nitrate 98% | [EINECS(EC#)]
208-060-1 | [Molecular Formula]
CH6N4O3 | [MDL Number]
MFCD00013028 | [Molecular Weight]
122.08 | [MOL File]
506-93-4.mol |
| Chemical Properties | Back Directory | [Appearance]
solid | [Melting point ]
213-215 °C(lit.)
| [Boiling point ]
227.45°C (rough estimate) | [density ]
1.44 | [vapor pressure ]
0Pa at 25℃ | [refractive index ]
1.4164 (estimate) | [storage temp. ]
Store below +30°C. | [solubility ]
water: soluble50mg/mL, clear to very slightly hazy, colorless | [form ]
crystalline powder | [pka]
10.2[at 20 ℃] | [color ]
White | [Odor]
Odorless | [PH]
4.9 (165g/l, H2O, 25℃) | [Stability:]
May explode if heated. May be shock sensitive. | [Water Solubility ]
130 g/L (20 ºC) | [Merck ]
14,4562 | [BRN ]
3596600 | [LogP]
-1.7 at 20℃ | [CAS DataBase Reference]
506-93-4(CAS DataBase Reference) | [EPA Substance Registry System]
506-93-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
O,Xn,Xi | [Risk Statements ]
R8:Contact with combustible material may cause fire.
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin .
R5:Heating may cause an explosion. | [Safety Statements ]
S17:Keep away from combustible material .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S15:Keep away from heat . | [RIDADR ]
UN 1467 5.1/PG 3
| [WGK Germany ]
1
| [RTECS ]
MF4350000
| [TSCA ]
Yes | [HazardClass ]
5.1 | [PackingGroup ]
III | [HS Code ]
29252000 | [Hazardous Substances Data]
506-93-4(Hazardous Substances Data) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Paraformaldehyde-->Ammonium nitrate-->Dicyandiamide-->Calcium cyanamide-->6-Ethyl-o-toluidine | [Preparation Products]
Bensulfuron methyl-->2-Aminopyrimidine-->Ethyl 5-[3-(4,6-dimethoxy pyrimidyl-2-yl)ureido]methylpyrazolyl-4-carboxylic ester-->Pirimiphos-methyl-->2,4-Diamino-5-pyrimidinemethanol-->2-Amino-4,6-dimethoxypyrimidine-->Guanidine carbonate-->Sulfometuron-methyl-->2,4-Diamino-5-(bromomethyl)pyrimidine-->Trimethoprim-->Diaveridine-->Sulfaguanidine-->Nitroguanidine-->2-Amino-4,6-dihydroxypyrimidine-->3-Amino-1,2,4-Triazole-->2-Nitroaminoimidazoline-->2,4-DIAMINOPYRIMIDINE-5-CARBONITRILE-->2,4-DIAMINOPYRIMIDINE-5-CARBOXYLIC ACID-->2,4,6-TRIAMINO-5-NITROPYRIMIDINE-->2,4-Diamino-6-hydroxypyrimidine-->4-Chloro-2,6-diaminopyrimidine-->2-Amino-4,6-dimethylpyrimidine-->Alkylimidazole |
| Hazard Information | Back Directory | [Hazard]
Strong oxidant, may ignite organic materials
on contact, may explode by shock or heat. | [Chemical Properties]
solid | [Uses]
Guanidine nitrate is the salt formed from guanidine and nitric acid and has been used as a high energy fuel used in gas generators and propellant applications. | [General Description]
Guanidine nitrate (GN) is an explosive used by the military and commercial sectors. Decomposition of guanidine nitrate has been investigated. | [Flammability and Explosibility]
Notclassified |
| Questions And Answer | Back Directory | [Guanidine Salt]
Nitrate guanidine��, guanidine hydrochloride, guanidine carbonate and guanidine phosphate are four common kinds of guanidine salts, can be used to produce sulfamidine, sulfadiazine sulfonamides and nitroguanidine, guanidine carbonate, and also used for photography materials and disinfectants. Industrial preparation of guanidine nitrate is through the reaction of dicyandiamide and ammonium nitrate.
Guanidine nitrate, also known as guanidine mononitrate, is colorless leave-like crystal when crystallized from water. It has moderate toxicity, Its molecular formula is H2NC (NH) NH2HNO3. It is white granules soluble in water and ethanol, slightly soluble in acetone. It has explosion decomposition at high temperatures. Its melting point is between 214 and 216 ° C. When it is rammed or exposed to high heat or fire, there is a risk of combustion explosion. And when mixed with sulfur, phosphorus and other reducing agents, it brings the danger of the formation of explosive mixtures. The mixtures of guanidine nitrate with nitro compounds, chlorates or strong acids are sensitive to vibration and friction and likely to cause explosion. It should be stored in shady and cool places and free from high temperature. Guanidine nitrate should be isolated from flammables, organic compounds, nitro compounds, chlorates or acids. The loading and unloading of guanidine nitrate should be carried out carefully and lightly with reinforcement pad to prevent knock and friction. And spilled guanidine nitrate should be immediately cleaned. Its fire extinguisher can be sand, foam, carbon dioxide and mist water.
Its United Nations No. (UN No.): 1467/5041-1,5.1 class, classified as oxidant.
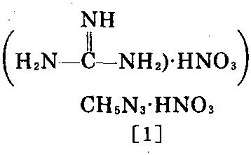
Image 1: Molecular structure of guanidine nitrate | [Physical and Chemical Properties]
White crystalline powder or granules. Soluble in water and alcohol but insoluble in acetone, benzene and ether. | [Category]
Oxidant | [Toxicological Information]
Toxicity Grading: moderate toxicity (WHO Class II)
Acute Toxicity:Oral-Rat LD50: 730 mg/kg; Oral-mouse LD50: 1028 mg/kg
Stimulation : Skin-Rabbit 500 mg ,Severe; Eyes-Rabbit 92 mg, Mild | [Applications]
Guanidine nitrate is not only used as the raw materials of imidacloprid (a pesticide) to prepare the next intermediate, nitroguanidine, it can also be used as the intermediates of a variety of sulfonylurea herbicides, such as bensulfuron-methyl, pyrazosulfuron-ethyl, chlorimuron-ethyl, etc. In addition, it is also used for the synthesis of sulfonamides medicine, explosives, photographic materials and disinfectants.In addition, it is also used to test guanidine salt in the complex acid. | [Synthesis]
The reaction of dicyandiamide and ammonium nitrate:
The condensation reaction dicyandiamide and ammonium nitrate in the ratio of 1: 2 is carried out at 120~210 ° C. The reaction product is crystallized, sliced to obtain finished product.
It also can be prepared by the reaction between calcium cyanamide and nitric acid. | [Handling and Storage]
It should be stored in low-temperature ventilated depot.
The loading and unloading of guanidine nitrate should be carried out carefully and lightly.
Always keep guanidine nitrate from organic materials, reducing agents, sulfur, phosphorus and flammables | [Flammability Hazard]
It is flammable at high temperature, and its combustion produces toxic nitrogen oxide fumes. | [Fire Fighting Measures]
Extinguishing Media:
Sand, carbon dioxide and mist water. | [Explosives hazard]
Explosive when rammed or exposed to high heat or fire.
Explosive when mixed with sulfur, phosphorus and other reducing agents. |
|