| Identification | More | [Name]
Phentolamine | [CAS]
50-60-2 | [Synonyms]
AKOS NCG1-0052
AURORA KA-7803
PHENTOLAMINE
2-((n-(m-hydroxyphenyl)-p-toluidino)methyl)-
2-((n-(m-hydroxyphenyl)-p-toluidino)methyl)-2-imidazolin
2-((n-(m-hydroxyphenyl)-p-toluidino)methyl)-2-imidazoline
2-(m-hydroxy-n-p-tolylanilinomethyl)-2-imidazoline
2-(n-(m-hydroxyphenyl)-p-toluidinomethyl)imidazoline
2-(n’-p-tolyl-n’-m-hydroxyphenylaminomethyl)-2-imidazoline
3-(((4,5-dihydro-1h-imidazol-2-yl)methyl)(4-methylphenyl)amino)-pheno
c7337ciba
dibasin
fentolamin
m-(n-(2-imidazolin-2-ylmethyl)-p-toluidino)-pheno
phenotolamine
phentalamine
regitine
regitipe
3-(N-2-imidazolin-2-ylmethyl-p-toluidino)phenol
3-[(4,5-dihydro-1h-imidazol-2-yl)methyl-(4-methylphenyl)-amino]phenol | [EINECS(EC#)]
200-053-1 | [Molecular Formula]
C17H19N3O | [MDL Number]
MFCD00242985 | [Molecular Weight]
281.35 | [MOL File]
50-60-2.mol |
| Chemical Properties | Back Directory | [Melting point ]
174-175° | [Boiling point ]
424.02°C (rough estimate) | [density ]
1.0510 (rough estimate) | [refractive index ]
1.6500 (estimate) | [storage temp. ]
Hygroscopic, -20°C Freezer, Under inert atmosphere | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
pKa 7.7 (Uncertain) | [color ]
Crystals | [Stability:]
Hygroscopic | [CAS DataBase Reference]
50-60-2(CAS DataBase Reference) |
| Questions And Answer | Back Directory | [description]
Phentolamine is an α-adrenergicreceptor antagonist approved for use by the U.S.Food and Drug Administration (FDA) in 1952. Approved uses of phentolamine currently include (1) diagnosis of pheochromocytoma,(2) treatment of hypertension in pheochromocytoma, and (3) prevention of tissue necrosis after norepinephrine extravasation. Anearly use of injectable phentolamine involved the management of impotence (erectile dysfunction).
Phentolamine is a short-acting, competitive antagonist at peripheral o-adrenergic receptors. It antagonizes both a and ozreceptors,thus blocking the actions of the circulating catecholamines epinephrine and norepinephrine. Phentolamine also stimulates β-adrenergic receptors in the heart and lungs. | [Pharmacology]
Analogues of the imidazoline adrenergic amines were among the first synthetic adrenergic blocking agents to be identified. Phentolamine is the only compound from this class that is still clinically available.
Phentolamine is a competitive non-selective α1- and α2-adrenergic receptor blocker of relatively short duration of action. It causes vasodilatation and a fall in blood pressure resulting from the blockade of both post-junctional vascular α1- and α2-adrenoceptors. It also antagonises the vasoconstrictor response to noradrenaline and adrenaline infusions. Enhanced neural release of noradrenaline due to presynaptic α2-blockade may contribute to the positive inotropic and chronotropic effects of Regitine on cardiac muscle.
| [Uses]
Phentolamine is a synthetic imidazoline with alpha-adrenergic antagonist activity. As a competitive alpha-adrenergic antagonist, phentolamine binds to alpha-1 and alpha-2 receptors, resulting in a decrease in peripheral vascular resistance and vasodilatation. This agent also may block 5-hydroxytryptamine (5-HT) receptors and stimulate release of histamine from mast cells.
Phentolamine is used mainly in the diagnosis of pheochromocytoma and to control or prevent paroxysmal hypertension immediately prior to or during pheochromocytomectomy.
Phentolamine has been used to treat hypertensive crises secondary to MAO inhibitor/sympathomimetic amine interactions and rebound hypertension on withdrawal of clonidine, propranolol or other antihypertensive agents.
| [clinical use]
The clinical effects of phentolamine include peripheral vasodilation and tachycardia.Vasodilation results from both direct relaxation of vascular smooth muscle and a blockade. The drug produces positive inotropic and chronotropic effects,leading to an increase in cardiac output.In smaller doses, the positive inotropic effect can predominate and raise blood pressure; in larger doses, peripheral vasodilation can mask the inotropic effect and lower blood pressure.These actions make phentolamine useful in treating hypertension caused by increased circulating levels of epinephrine and norepinephrine, as occurs in pheochromocytoma.
The effects of phentolamine in treating impotence are mediated by α-adrenergic blockade in penile blood vessels. Actions of the drug cause relaxation of the trabecular cavernous smooth muscles and dilation of the penile arteries; this increases arterial blood flow into the corpus cavernosa and subsequently causes an erection. Phentolamine is administered IV or IM but can be injected subcutaneously to prevent local tissue necrosis when vasoconstrictor drugs extravasate. The pharmacokinetics of phentolamine is largely unknown;10% of a parenteral dose is excreted in the urine unchanged.
| [Carcinogenicity / mutagenicity]
Experimental data have established that phentolamine lacks mutagenic potential in bacteria, and does not induce chromosomal aberrations in mammalian somatic cells in vivo. Long-term carcinogenicity studies have not been conducted with phentolamine. | [Overdosage]
No deaths due to acute poisoning with phentolamine have been reported.
Overdosage with parenterally administered phentolamine is characterized chiefly by cardiovascular disturbances, such as arrhythmias, tachycardia, hypotension, and possibly shock. In addition, the following might occur: excitation, headache, sweating, pupillary contraction, visual disturbances, nausea, vomiting, diarrhea, or hypoglycemia.
There is no specific antidote; treatment consists of appropriate monitoring and supportive care. Substantial decreases in blood pressure or other evidence of shock-like conditions should be treated vigorously and promptly.
|
| Hazard Information | Back Directory | [Originator]
Regitine, Ciba, US ,1952 | [Definition]
ChEBI: A substituted aniline that is 3-aminophenol in which the hydrogens of the amino group are replaced by 4-methylphenyl and 4,5-dihydro-1H-imidazol-2-ylmethyl groups respectively. An alpha-adrenergic antagonist, it is used fo
the treatment of hypertension. | [Indications]
Human erectile tissue has a population of membrane
receptors that are predominantly of the �-adrenoceptor
subtype. Phentolamine (Vasomax) is a nonselective �-
adrenoceptor blocking agent (see Chapter 11), and like
other such agents, it has been used to treat ED.
Nonselective adrenoceptor antagonists may provoke a
reflex that increases both sympathetic outflow and the
release of norepinephrine. | [Manufacturing Process]
199.24 parts of N-(p-methylphenyl)-m'-hydroxyphenylamine and 77.52 parts of 2-chloromethylimidazoline hydrochloride are heated for sixteen hours in an oil bath having a temperature of 150°C, while stirring and introducing a current of nitrogen. The viscous contents of the flask are then cooled to about 100°C, mixed with 400 parts by volume of hot water, and stirred for a short time.
After further cooling to about 60°C, 200 parts by volume of water and 500 parts by volume of ethyl acetate at 60°C are added, and the aqueous layer is separated. The excess of starting material may be recovered from the ethyl acetate.
The aqueous portion is chilled in a cooling chamber at -10°C, whereupon the hydrochloride of 2-[N-(p-methylphenyl)-N-(m'-hydroxyphenyl)-aminomethyl]imidazoline crystallizes. Upon being concentrated and cooled the mother liquor yields a further quantity of the hydrochloride. The combined quantities of hydrochloride are treated with a small quantity of cold water, dried with care, and washed with ethyl acetate. The product is then crystallized from a mixture of alcohol and ethyl acetate, and there is obtained a hydrochloride melting at 239°-240°C. | [Brand name]
Regitine
(Novartis) [Name previously used: Phentolamine Methanesulfonate.]. | [Therapeutic Function]
Adrenergic blocker | [General Description]
Phentolamine isthe more effectiveα -blocker. | [Mechanism of action]
Its mechanism of action is as an α-adrenergic antagonist of both α1- and α2-receptors, causing vasodilation and reduction in peripheral resistance. When administered by intracavernosal injection, it is thought to cause relaxation of the cavernous smooth muscles and vasodilation of the penile arteries. This results in increased arterial blood flow into the corpus cavernosa as well as swelling and elongation of the penis. Venous outflow also is reduced, possibly as a result of increased venous resistance. Phentolamine is slowly released into venous circulation with minimal, if any, systemic effects. Time to peak effect is within 10 minutes, and duration of action when used with papaverine is 1 to 6 hours. | [Side effects]
Side effects of phentolamine are dose related. It
may cause orthostatic hypotension, reflex tachycardia,
cardiac arrhythmias, and rarely, myocardial infarction.
Phentolamine also may reduce sperm motility in vitro. | [Safety Profile]
Poison by subcutaneous, intravenous, and intraperitoneal routes. Moderately toxic by ingestion. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx. | [Synthesis]
Phentolamine, 2-[[N-(3??-hydroxyphenyl)-para-toluidion]methyl]-2-
imidazoline (12.2.8), is synthesized by alkylation of 3-(4-methylanilino)phenol using
2-chloromethylimidazoline [36, 37].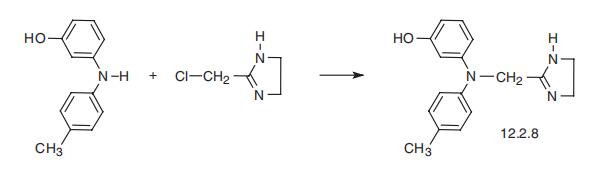
|
|
|