| Identification | More | [Name]
2-Methylsuccinic acid | [CAS]
498-21-5 | [Synonyms]
sc5276
PYROTARTARIC ACID
Methyl-Butanediolc
METHYLSUCCINIC ACID
2-Methylsuccinic aci
2-Methylsuccinic acid
methyl-butanedioicaci
methyl-Butanedioicacid
Methylbutanedioic acid
Succinic acid, methyl-
Methylsuccinicacid,99%
2-Methylbutanedioicacid
Butanedioicacid,methyl-
2-Methylbutanedioic acid
(+/-)-METHYLSUCCINIC ACID
DL-Methylsuccinic acid,99%
,2-Propanedicarboxylic acid
1,2-Propanedicarboxylic acid
Propane-1,2-dicarboxylicacid
2-Methylbutane-1,4-dioic acid
(plus-minus)-methylsuccinic acid
Methyl-Butanedioic acid Methylsuccinic acid Pyrotartaric acid | [EINECS(EC#)]
207-857-1 | [Molecular Formula]
C5H8O4 | [MDL Number]
MFCD00002659 | [Molecular Weight]
132.11 | [MOL File]
498-21-5.mol |
| Chemical Properties | Back Directory | [Appearance]
white to beige crystalline powder | [Melting point ]
110-115 °C (lit.) | [alpha ]
[α]D20 -1~+1° (c=5, C2H5OH) | [Boiling point ]
164.01°C (rough estimate) | [density ]
1.0951 (rough estimate) | [refractive index ]
1.4240 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Chloroform (Slightly), DMSO (Sparingly), Methanol (Slightly) | [form ]
Solid | [pka]
4.13(at 25℃) | [color ]
White to Off-White | [PH]
3.62(1 mM solution);3.08(10 mM solution);2.57(100 mM solution) | [Stability:]
Stable. Combustible. Incompatible with bases, oxidizing agents, reducing agents. | [Water Solubility ]
Soluble in water. | [BRN ]
1722946 | [Uses]
Organic synthesis.
| [CAS DataBase Reference]
498-21-5(CAS DataBase Reference) | [NIST Chemistry Reference]
Butanedioic acid, methyl-(498-21-5) | [EPA Substance Registry System]
498-21-5(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [TSCA ]
Yes | [HS Code ]
29171900 |
| Hazard Information | Back Directory | [General Description]
White or yellowish crystals or beige powder. | [Reactivity Profile]
Carboxylic acids, such as METHYL SUCCINIC ACID(498-21-5), donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in METHYL SUCCINIC ACID(498-21-5) to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. | [Air & Water Reactions]
Water soluble. | [Health Hazard]
ACUTE/CHRONIC HAZARDS: This compound may be harmful by inhalation, ingestion or skin absorption. When heated to decomposition it emits toxic fumes of carbon monoxide and carbon dioxide. | [Fire Hazard]
Flash point data for this compound are not available; however, METHYL SUCCINIC ACID is probably combustible. | [Description]
Methylsuccinic acid is a normal metabolite found in human fluids and is an intermediate metabolite in the breakdown of fatty acids. Increased urinary levels of methylsuccinic acid (together with ethylmalonic acid) are the main biochemical measurable features in ethylmalonic encephalopathy. | [Chemical Properties]
white to beige crystalline powder | [Definition]
ChEBI: 2-methylbutanedioic acid is a dicarboxylic acid that is butanedioic acid substituted at position 2 by a methyl group. It is a conjugate acid of a methylsuccinate. | [Preparation]
A common method employed for the synthesis of alkanedioic acids involves the oxidation of lactone derivatives. However, in the case of y-butyrolactones, the oxidations have proven particularly challenging due to the stability of the five-membered lactones. A successful approach to this type of oxidative preparation involves the carboxylation of γ-butyrolactone using carbon monoxide and hydrogen fluoride-antimony(V) fluoride superacid containing an excess of antimony(V) fluoride to afford 2-methylsuccinic acid.
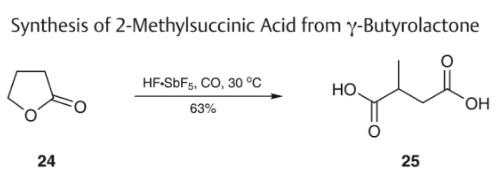
Synthesis of 2-Methylsuccinic Acid from y-Butyrolactone | [Origin]
2-Methylsuccinic acid is a natural product found in African aloe vera. | [Synthesis]
It can be prepared by partial hydrogenation of itaconic acid over Raney nickel. Alternatively, hydrocyanation of ethyl crotonate affords an intermediate, which converts to 2-methylsuccinic acid after hydrolysis of the ester and nitrile substituents. | [First aid]
Eyes: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
Skin: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
Inhalation: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
Ingestion: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital.
| [Purification Methods]
Crystallise the acid from water. [Beilstein 2 IV 1948.] |
|
|