| Identification | More | [Name]
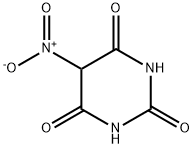
5-NITROBARBITURIC ACID | [CAS]
480-68-2 | [Synonyms]
5-NITRO-2,4,6-TRIHYDROXYPYRIMIDINE
5-NITROBARBITURIC ACID
AKOS 89968
AURORA KA-3829
DILITURIC ACID
NITROBARBITURIC ACID
2,4,6(1H,3H,5H)-Pyrimidinetrione,5-nitro-
5-nitro-2,4,6(1h,3h,5h)-pyrimidinetrione
5-Nitro-2,4,6-(1H,3H.5H)-pyrimidinetrione
5-nitro-barbituricaci
5-Nitrobarbiuricacid
6(1h,3h,5h)-pyrimidinetrione,5-nitro-4
5-Nitrobarbitursαure
5-Nitro-hexahydropyrimidine-2,4,6-trione | [EINECS(EC#)]
207-557-0 | [Molecular Formula]
C4H3N3O5 | [MDL Number]
MFCD00006667 | [Molecular Weight]
173.08 | [MOL File]
480-68-2.mol |
| Chemical Properties | Back Directory | [Appearance]
Prisms and leaflets from water.Slightly soluble in water; soluble in
alcohol and sodium hydroxide solution; insoluble
in ether. | [Melting point ]
180.5°C | [Boiling point ]
303.69°C (rough estimate) | [density ]
1.79±0.1 g/cm3(Predicted) | [refractive index ]
1.5000 (estimate) | [storage temp. ]
Store at -20°C | [form ]
Solid | [pka]
-1.70±0.20(Predicted) | [color ]
Off-white to light yellow | [Merck ]
6585 | [Uses]
Microreagent for potassium. | [CAS DataBase Reference]
480-68-2(CAS DataBase Reference) | [EPA Substance Registry System]
480-68-2(EPA Substance) |
| Questions And Answer | Back Directory | [synthesis]
5-Nitrobarbituric acid can be obtained by nitration of barbituric acid with fuming nitric acid below 40°C with a yield of 85-90%. |
| Safety Data | Back Directory | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RTECS ]
CQ7300000 | [HazardClass ]
IRRITANT | [HS Code ]
29335400 |
| Hazard Information | Back Directory | [Chemical Properties]
Prisms and leaflets from water.Slightly soluble in water; soluble in
alcohol and sodium hydroxide solution; insoluble
in ether. | [IC 50]
HSV-1: 1.7 μM (IC50) | [Purification Methods]
Crystallise diliuric acid from water as the trihydrate (m 180-181o (dec). Drying over 70% H2SO4 converts the trihydrate to the dihydrate [Loeffler & Moore J Am Chem Soc 70 3650 1948]. [Beilstein 24 H 474, 24 II 273, 24 III/IV 1882.] |
|
|