| Identification | Back Directory | [Name]
N-(2-chloropyriMidin-4-yl)-N,2,3-triMethyl-2H-indazol-6-aMine | [CAS]
444731-75-3 | [Synonyms]
BNKY007-PZ02
Pazopanib Impurity 22
Pazopanib intermediate
Pazopanib ( PAZ ) N - Methyl compound
N-(2-chloropyrimidin-4-yl)-N,2,3-trimethylindazol-6-amine
N-(2-chloropyriMidin-4-yl)-N,2,3-triMethyl-2H-indazol-6-aMine
N-(2-chloro-4-pyriMidinyl)-N,2,3-triMethyl-2H-indazol-6-aMine
N-(2-chloropyrimidin-4-yl)-N,2,3-trimethyl-1H-indazol-6-amine
N-(2-chloropyrimidin-4-yl)-N,2,3-trimethyl-2H-indazol-6-amin...
2H-Indazol-6-aMine, N-(2-chloro-4-pyriMidinyl)-N,2,3-triMethyl-
2H-indazole-6-aMine�����,N-(2-chloro
-4-pyrimidinyl)-N,2,3-trimethyl-
N-(2-ChloropyriMidin-4-yl)-N-Methyl-2,3-diMethyl-2H-indazol-6-aMine
2,3-dimethyl-N-(2-chloropyrimidin-4-yl)-N-methyl-2H-indazol-6-amine
(2-Chloro-pyrimidin-4-yl)-(2,3-dimethyl-2H-indazol-6-yl)-methyl-amine
6-[N-(2-chloro-4-pyrimidinyl)methylamino]-2,3-dimethyl-2H-indazole,96%
N-(2-chloropyrimidine-4-yl) -N-methyl-2, 3-dimethyl-2H-indazol-6-amine
6-[N-(2-chloro-4-pyriMidinyl)MethylaMino]-2,3-diMethyl-2H-indazole, 96%
TIANFU-CHEM - N-(2-chloropyriMidin-4-yl)-N,2,3-triMethyl-2H-indazol-6-aMine
N-(2- chloropyrimidine-4- group)- N- methyl-2,3-dimethyl-2 H- indazole-6-amine
N-(2-chloropyriMidin-4-yl)-N,2,3-triMethyl-2H-indazol-6-aMine ISO 9001:2015 REACH | [EINECS(EC#)]
810-050-2 | [Molecular Formula]
C14H14ClN5 | [MDL Number]
MFCD12923006 | [MOL File]
444731-75-3.mol | [Molecular Weight]
288 |
| Chemical Properties | Back Directory | [Melting point ]
167-173℃ | [Boiling point ]
524.4±35.0 °C(Predicted) | [density ]
1.33±0.1 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Very Slightly) | [pka]
2.82±0.30(Predicted) | [Stability:]
Hygroscopic | [CAS DataBase Reference]
444731-75-3 |
| Hazard Information | Back Directory | [Chemical Properties]
Light Beige to Beige Solid | [Uses]
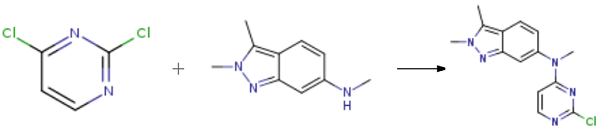
N-(2-Chloro-4-pyrimidinyl)-N,2,3-trimethyl-2H-indazol-6-amine is an impurity of Pazopanib (P210925), an oral angiogenesis inhibitor targeting VEGFR and PDGFR. | [Synthesis]
A flask was charged with N,2,3-trimethyl-2H-indazol-6-amine (1 eq.), 2,4-dichloropyrimidine (1.5 eq.), sodium bicarbonate (2 eq.) and N, N-dimethylformamide. The reaction mixture was stirred at 85 °C until completion of the reaction. Then, water was added and stirred for 3 h. Product crystals were collected by filtration, washed with water, and then dried to get N-(2-chloropyriMidin-4-yl)-N,2,3-triMethyl-2H-indazol-6-aMine as off-white/beige powder (Yield: 97%).
|
|
|