| Identification | More | [Name]
3-ISOCHROMANONE | [CAS]
4385-35-7 | [Synonyms]
1,4-DIHYDRO-3H-2-BENZOPYRAN-3-ONE
3-ISOCHROMANONE
ISOCHROMAN-3-ONE
3-ISOCHROMANONE 99+%
3,4-Dihydro-1H-2-benzopyran-3-one | [EINECS(EC#)]
224-493-9 | [Molecular Formula]
C9H8O2 | [MDL Number]
MFCD00043005 | [Molecular Weight]
148.16 | [MOL File]
4385-35-7.mol |
| Chemical Properties | Back Directory | [Melting point ]
80-82 °C (lit.) | [Boiling point ]
130 °C / 1mmHg | [density ]
1.196±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Chloroform (Slightly, Heated, Sonicated), Methanol (Slightly) | [form ]
Solid | [color ]
White to Pale Beige | [BRN ]
123692 | [InChI]
InChI=1S/C9H8O2/c10-9-5-7-3-1-2-4-8(7)6-11-9/h1-4H,5-6H2 | [InChIKey]
ILHLUZUMRJQEAH-UHFFFAOYSA-N | [SMILES]
C1(=O)OCC2=CC=CC=C2C1 | [CAS DataBase Reference]
4385-35-7(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [HS Code ]
2932990090 |
| Hazard Information | Back Directory | [Chemical Properties]
Lamellae | [Uses]
3-Isochromanone may be used as starting reagent in the synthesis of of BDPBI (7-bromo-1,4-dihydro-2-phenyl-4,4-bis(4-pyridinylmethyl)2H-isoquinolin-3-one dihydrochloride). | [Production Methods]
3-Isochromanone is a well-known compound, and several methods for its preparation are described in the chemical literature. For example, it can be prepared by (i) the Baeyer-Villiger oxidation of 2-indanone using hydrogen peroxide in sulphuric acid and acetic anhydride or using 3-chloroperoxybenzoic acid combined with trifluoroacetic acid; (ii) from 2-methoxycarbonylmethylbenzoic acid by (a) treatment with ethyl chloroformate in triethylamine and (b) sodium borohydride; or (iii) from isochroman-3-ol and chromium trioxide. It is also known to prepare 3-isochromanone by the bromination of o-tolylacetic acid with N-bromosuccinimide followed by ring closure by boiling the 2-bromomethylphenylacetic acid so formed with potassium hydroxide in ethanol. | [Reactions]
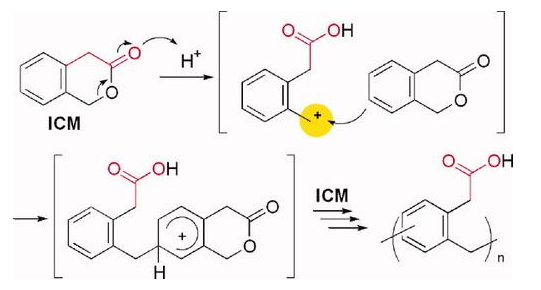
3-Isochromanone (ICM) is an aromatic lactone. ICM can be synthesised by cationic ring opening polymerisation to produce a polyphenylene containing carboxylic acid molecules in the side chain. the polymerisation of ICM proceeds through the formation of a benzyl cationic intermediate and its successive Friedel-Crafts reactions[1].
 | [Synthesis Reference(s)]
Journal of the American Chemical Society, 102, p. 4193, 1980 DOI: 10.1021/ja00532a034
Tetrahedron Letters, 36, p. 8123, 1995 DOI: 10.1016/0040-4039(95)01692-B | [General Description]
3-Isochromanone has been reported to be isolated from the fungus Nigrospora sp. PSU-F12. An improved Knoevenagel condensation of 3-isochromanone with aromatic aldehydes has been achieved by microwave irradiation on solid supports in the presence of various catalysts. Synthesis of 3-isochromanone via Beayer-Villiger rearrangement has been reported. | [Synthesis]
o-Tolylacetic acid (50g at 98% strength, 0.326 mol) in fluorobenzene (76.7g) was dried by azeotropic distillation and cooled to 60°C. AIBN (2.13g, 0.013 mol) was added in one portion, followed by sulphuryl chloride (49.8g at 97% strength, 0.358 mol) over 3 hours while maintaining the temperature at 60-62°C. A small sample was removed from the mixture, diluted with more solvent, and analyzed by GC. This showed the presence of 10% o-tolylacetic acid starting material. A 20% aqueous solution of potassium bicarbonate (60.6g, 0.121 mol) was added slowly to the reaction mixture, followed by potassium iodide (0.22g) and then, slowly, by solid potassium bicarbonate (20.95g, 0.209 mol). Stirring was continued for 1 hour at 60°C. Further solid potassium bicarbonate (7.9g) was added at 60°C, and stirring continued for another 15 minutes. The reaction mixture was left to stand and cool to ambient temperature overnight under nitrogen. It was then warmed to 65°C, separating the aqueous and organic layers. The organic layer was diluted with fluorobenzene (50 ml), used to wash the separator, and dried by azeotropic distillation. The product precipitated on cooling after cyclohexane was added slowly at 60-65°C. The temperature was reduced to around 5°C, and the solids were filtered and sucked dry to give 27.18g (100% wt) 3-isochromanone; 56.3% yield; mp 79-80°C. | [References]
[1] AKANE SUZUKI; Takeshi E; Atsushi Sudo. Cationic ring-opening polymerization of 3-isochromanone through formation of benzyl cationic intermediate and its Friedel-Crafts reaction[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2009. DOI:10.1002/pola.23318. |
|
|