| Identification | Back Directory | [Name]
Belinostat (PXD101) | [CAS]
414864-00-9 | [Synonyms]
PXD101
PX105684
NSC726630
BelioMstat
Belinostat
BENLINOSTAT
Belinostat-D5
Belinostat-13C6
BelioMstat
PXD101
PXD101, BelioMstat
Belinostat(PXD101)
N-HYDROXY-3-(3-PHENYLSULFAMOYLPHENYL)ACRYLAMIDE
(E)-N-hydroxy-3-(3-(N-phenylsulfaMoyl)phenyl)acrylaMide
N-HYDROXY-3-[3-[(PHENYLAMINO)SULFONYL]PHENYL]-2-PROPENAMIDE
2-PropenaMide, N-hydroxy-3-[3-[(phenylaMino)sulfonyl]phenyl]-
N-Hydroxy-3-(3-phenylsulfamoylphenyl)acrylamide Belinostat | [Molecular Formula]
C15H14N2O4S | [MDL Number]
MFCD08064035 | [MOL File]
414864-00-9.mol | [Molecular Weight]
318.35 |
| Chemical Properties | Back Directory | [Melting point ]
142 - 145°C | [density ]
1.427±0.06 g/cm3(Predicted) | [storage temp. ]
Amber Vial, Refrigerator, Under inert atmosphere | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
8.31±0.10(Predicted) | [color ]
Off-White to Pale Orange | [Stability:]
Light Sensitive |
| Hazard Information | Back Directory | [Usage]
Belinostat is a novel histone deacetylase 3 selective inhibitor, which protects the β cells from cytokine-induced apoptosis. | [Description]
Belinostat is a drug which was developed by Spectrum Pharmaceuticals
and is currently marketed by Onxeo as Beleodaq®. The
drug, which received fast track designation by the United States
Food and Drug Administration (US FDA) and was approved for
the treatment of hematological malignancies and solid tumors
associated with peripheral T-cell lymphoma (PTCL) in 2014, is a
histone deacetylase (HDAC) inhibitor and is the third such treatment
to receive accelerated approval for PTCL, the others being
vorinostat (Zolinza®) and pralatrexate (Folotyn®). Although belinostat
was not yet approved in Europe as of August 2014, the
compound exhibits a safety profile considered to be acceptable
for HDAC inhibitors–less than 25% of patients reported adverse
effects and these most frequently were nausea, fatigue, pyrexia,
anemia, and emesis. | [Uses]
Belinostat is a novel histone deacetylase 3 selective inhibitor, which protects the β cells from cytokine-induced apoptosis. | [Definition]
ChEBI: A hydroxamic acid-type histone deacetylase (HDAC) inhibitor with antineoplastic activity. | [Synthesis]
Commercially available 3-bromobenzenesulfonyl chloride (41)
was reacted with aniline in the presence of aqueous sodium carbonate
to deliver sulfonamide 42 in 94% yield. Next, this aryl bromide
was subjected to a Heck reaction involving ethyl acrylate to
give rise to cinnamate ester 43, which was immediately saponified
under basic conditions and acidic workup to furnish the corresponding
acid 44. This acid was activated as the corresponding acid
chloride prior to subjection to hydroxylamine under basic conditions
to form the hydroxamic acid, which was then recrystallized
from an 8:1 ethanol/water mixture in the presence of a catalytic
amount of sodium bicarbonate to furnish crystalline belinostat
(VI) in 87% overall yield from acid 44.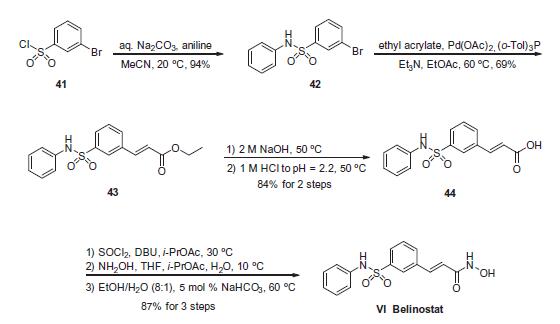
| [target]
pan-HDAC | [storage]
Store at -20°C |
|
|