| Identification | More | [Name]
4-IODOBUTYL ACETATE | [CAS]
40596-44-9 | [Synonyms]
4-IODOBUTYL ACETATE
1-ACETOXY-4-IODOBUTANE
4-iodobutyl acetate 96%
4-iodo-1-butanol acetate
4-ACETOXY-BUTAN-1-IODIDE
1-Butanol, 4-iodo-, 1-acetate
4-Iodobutylacetate, 85%, tech.
ACETIC ACID 4-IODO-BUTYL ESTER
4-Iodobutyl acetate, 97%, stab
4-IODOBUTYL ACETATE, TECH., 85%
4-Iodobutyl acetate,stab. with copper
4-Iodobutylacetate1-Acetoxy-4-iodobutane
4-Iodobutyl acetate, 97%, stab. with copper
4-Iodobutyl acetate, stabilized with copper
4-Iodobutyl acetate, 96%, stab. with copper | [Molecular Formula]
C6H11IO2 | [MDL Number]
MFCD00010664 | [Molecular Weight]
242.05 | [MOL File]
40596-44-9.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [WGK Germany ]
3
| [HS Code ]
29153900 |
| Hazard Information | Back Directory | [Chemical Properties]
CLEAR DARK BROWN LIQUID | [Uses]
4-Iodobutyl acetate is used in the preparation of tricyclohexyl(acetoxybutyl))phosphonium iodide, tri-n-hexyl(acetoxybutyl))phosphonium iodide | [Synthesis Reference(s)]
Synthetic Communications, 24, p. 951, 1994 DOI: 10.1080/00397919408020770 | [Synthesis]
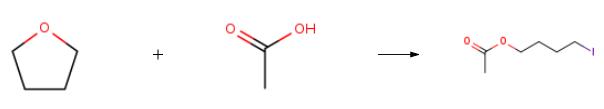
The synthesis of 4-IODOBUTYL ACETATE is as follows:Acetic acid (0.30mmol, 1.0 equivalent), CuI (0.03mmol, 10.0mol%, 5.7mg) and NaI (0.60mmol, 2.0 equivalent, 89.9mg) were added to a 10mL Schlenk tube equipped with a magnetic stirring device. Vacuum the flask with a pump and backfill it three times with nitrogen. Then, 1.0 mL tetrahydrofuran (12.30 mmol, 41.0 equivalence, 1 mL, c=0.30 M), deionized water (0.45 mmol, 1.5 equivalence) and TMSCF3 (0.36 mmol, 1.2 equivalence) were added under N2 atmosphere. The mixture is stirred at 150°C (thermostatic oil bath pot) for 12 hours. After cooling to room temperature, dilute the mixture with ethyl acetate (15 mL), wash with water and brine, and dry on anhydrousNa2SO4. After Extraction by EtOAc (5 mL x 3), the combined organic layer was dehydrated with anhydrousNa2SO4 and concentrated under reduced pressure. The crude product is purified by rapid chromatography on silica gel to obtain alkyl iodide.
 |
|
| Company Name: |
Alfa Aesar
|
| Tel: |
400-6106006 |
| Website: |
http://chemicals.thermofisher.cn |
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|