| Identification | More | [Name]
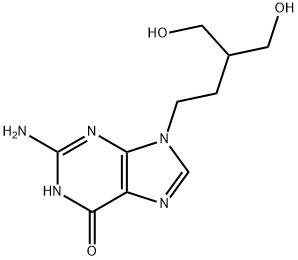
2-Amino-9-[4-hydroxy-3-(hydroxymethyl)butyl]-3,9-dihydropurin-6-one | [CAS]
39809-25-1 | [Synonyms]
2-AMINO-1,9-DIHYDRO-9-[4-HYDROXY-3-(HYDROXYMETHYL)BUTYL]-6H-PURIN-6-ONE
2-AMINO-1,9-DIHYDRO-9-[4-HYDROXY-3-(HYDROXYMETHYL)BUTYL]-6H-PURIN-6-ONE-D4
2-amino-9-[4-hydroxy-3-(hydroxymethyl)butyl]-3,9-dihydropurin-6-one
PENCICLOVIR
PENCICLOVIR-D4
Penciceovir
9-[4-hychoxy-3-(hydvoxymethyl)butyl]guanine
BRL-39123
BRL-39123A
PCV
Vectavir
6H-Purin-6-one, 2-amino-1,9-dihydro-9-[4-hydroxy-3-(hydroxymethyl)butyl]-
9-[4-Hydroxy-3-(hydroxymethyl)butyl]guanine
2-[2-[(2-Amino-1,6-dihydro-6-oxo-9H-purin)-9-yl]ethyl]propane-1,3-diol
2-Amino-9-(4-hydroxy-3-hydroxymethylbutyl)-9H-purin-6(1H)-one
2'-Carba-ganciclovir | [EINECS(EC#)]
663-371-3 | [Molecular Formula]
C10H15N5O3 | [MDL Number]
MFCD00866931 | [Molecular Weight]
253.26 | [MOL File]
39809-25-1.mol |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
6-Chloroguanine-->Triphenylphosphine-->Sodium hydroxide-->Carbon tetrabromide-->Hydrochloric acid-->Lithium Aluminum Hydride-->p-Toluenesulfonic acid monohydrate-->9H-Purin-2-amine, 6-chloro-9-[2-(2,2-dimethyl-1,3-dioxan-5-yl)ethyl]--->5-(2-BROMOETHYL)-2,2-DIMETHYL-1,3-DIOXANE-->2-(2,2-Dimethyl-1,3-dioxan-5-yl)ethanol-->2-(hydroxyMethyl)butane-1,4-diol-->9-(4-Acetoxy-3-acetoxymethylbutyl)-2-amino-6-chloropurine-->1,3-Propanediol, 2-[2-(2-amino-6-chloro-9H-purin-9-yl)ethyl]--->Triethyl 1,1,2-ethanetricarboxylate | [Preparation Products]
6H-PURIN-6-ONE, 1,9-DIHYDRO-9-[3-[[(4-METHOXYPHENYL)DIPHENYLMETHOXY]METHYL]-4-[[(4-METHYLPHENYL)SULFONYL]OXY]BUTYL]-2-[[(4-METHOXYPHENYL)DIPHENYLMETHYL]AMINO]--->BRL 42222 |
| Hazard Information | Back Directory | [Description]
Vectavir was launched in the UK for herpes labialis. Penciclovir is
synthetically available by two routes of four steps each from 2-
(hydroxymethyl)butane-l,4-diol and is active against HSV-1, HSV-2 VZV but has
limited activity against CMV. Vectavir is an acyclic guanosine analog that acts as a
competitive inhibitor of DNA polymerase. It is a metabolic product of famcyclovir that
is preferentially phosphorylated by viral infected cells (by thymidine kinases) over
normal cells. The triphosphate has a low activity against cellular DNA polymerase
which is one possible explanation for its low toxicity. While its spectrum of activity is
similar to acyclovir, it is longer acting because its triphosphate is 20 times more
stable and is not metabolized. | [Chemical Properties]
White Cyrstalline Solid | [Originator]
SmithKline Beecham (UK) | [Uses]
A deuterated version of Penciclovir, an antiviral | [Definition]
ChEBI: A member of the class of 2-aminopurines that is guanine in which the hydrogen at position 9 is substituted by a 4-hydroxy-3-(hydroxymethyl)but-1-yl group. An antiviral drug, it is administered topically for treatment of herpes labialis. A prodrug, famciclo
ir, is used for oral administration. | [Indications]
Penciclovir has activity against HSV-1, HSV-2,
VZV, and HBV. After oral administration, famciclovir is
converted to penciclovir by first-pass metabolism.
Penciclovir has a mechanism of action similar to that of
acyclovir. It is first monophosphorylated by viral thymidine
kinase; then it is converted to a triphosphate by
cellular kinases. | [Manufacturing Process]
To a suspension of lithium aluminum hydride (2.87 g, 76 mmol) in tetrahydrofuran (125 ml), a solution of triethyl 1,1,2-ethanetricarboxylate (9.2 ml, 9.85 g, 40 mmol) in tetrahydrofuran (25 ml) was added dropwise with stirring over 2 hours. The inorganic salts were filtered off and washed with ethanol (100 ml). The filtrate and washings were combined and the solvent was evaporated under reduced pressure to afford a colourless oil (4.85 g). To a suspension of this oil in acetone (100 ml) 2,2-dimethoxypropane (25 ml) and p-toluenesulphonic acid monohydrate (2.3 g, 12 mmol) were added. The mixture was stirred for 1 hour. The resulting solution was neutralised with Amberlite IR 45 (methanol washed), filtered and the solvent evaporated under reduced pressure. The residue was purified by column chromatography on silica gel, eluting with chloroform-methanol mixtures (40:1 and 25:1) to afford 5-(2-hydroxyethyl)-2,2-dimethyl-1,3-dioxan as a colourless liquid (3.01 g, 47%).
To an ice-cooled solution of 5-(2-hydroxyethyl)-2,2-dimethyl-1,3-dioxan (1.92 g, 12 mmol) and carbon tetrabromide (7.96 g, 24 mmol) in dimethylformamide (100 ml) triphenylphosphine (6.30 g, 24 mmol) was added and the solution was left at 4°C overnight. To this solution methanol (20 ml) was added and the solvent was then evaporated under reduced pressure. The residue was purified by column chromatography on silica gel, eluting with hexane-acetone (12:1) to afford 5-(2-bromoethyl)-2,2-dimethyl1,3-dioxan as a clear colourless liquid (0.89 g, 40%).
To a solution of 5-(2-bromoethyl)-2,2-dimethyl-1,3-dioxan (0.75 g, 3.7 mmol) in dry dimethylformamide (12 ml) 2-amino-6-chloropurine (0.68 g, 4.0 mmol) and then anhydrous potassium carbonate (0.83, 6.0 mmol) were added. The solution was stirred at room temperature for 5 hours and left at 4°C
overnight. The solution was filtered and the solvent removed. The residue was purified by column chromatography on silica gel, eluting with chloroformmethanol mixtures (80:1 and 60:1) to afford 2-amino-6-chloro-9-[2-(2,2dimethyl-1,3-dioxan-5-yl)ethyl]purine as a white crystalline solid (0.74 g, 64%), melting point 125°-126°C.
2-Amino-6-chloro-9-[2-(2,2-dimethyl-1,3-dioxan-5-yl)-ethyl]purine (0.59 g, 1.9 mmol) in hydrochloric acid (1.0 M, 4 ml) was stirred at 60°C for 24 hours. The solution was diluted with water and neutralised with Amberlite IR 45. The mixture was filtered, the resin washed with water and the solvent evaporated under reduced pressure. The residue was recrystallised from water to afford 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (238 mg, 49%), melting point 275°-277°C.
| [Brand name]
Denavir (SmithKline Beecham). | [Therapeutic Function]
Antiviral | [Acquired resistance]
Penciclovir is inactive against thymidine kinase-deficient
strains of HSV. | [Pharmaceutical Applications]
A synthetic acyclic purine nucleoside analog, usually administered
orally as the diacetyl ester, famciclovir, which acts as
a prodrug undergoing rapid first-pass metabolism to release
the active compound in vivo. The parent compound has
virtually no oral bioavailability, but is supplied as a topical
formulation. | [Pharmacokinetics]
Oral absorption, penciclovir: 5%
famciclovir: 77%
Cmax famciclovir 250 mg oral: 1.6 mg/L after 0.5–1.5 h
famciclovir 500 mg oral: 3.3 mg/L after 0.5–1.5 h
famciclovir 750 mg oral: 5.1 mg/L after 0.5–1.5 h
Plasma half-life: 2.1–2.7 h
Volume of distribution: c. 1.5 L/kg
Plasma protein binding: <20%
Following absorption famciclovir is converted rapidly by
enzyme-mediated deacetylation and oxidation to penciclovir.
Food does not lead to any significant change in the availability
or elimination.
The pharmacokinetics in elderly subjects are similar to
those seen in younger subjects, although small increases in
AUC and plasma half-lives were seen, consistent with slightly
decreased renal clearance.
Renal excretion is the major route of elimination, 50–60%
of an oral dose being recovered in the urine. After intravenous
infusion, about 70% is excreted unchanged in the urine.
After oral administration of famciclovir, penciclovir accounts
for 82% of urinary drug-related material. The remainder
includes metabolites, of which the largest is the 6-deoxy precursor
of penciclovir. Renal clearance exceeds glomerular filtration,
indicating renal tubular secretion. | [Clinical Use]
Herpes zoster and genital herpes
Orolabial herpes (topical) | [Clinical Use]
Penciclovir is approved as a topical formulation for the
treatment of herpes labialis. In immunocompetent individuals,
penciclovir shortens the duration of lesion presence
and pain by approximately half a day when it is initiated
within an hour of lesion development and applied
every 2 hours during waking hours for 4 days. | [Side effects]
In clinical trials the incidence of adverse events after famciclovir,
aciclovir and placebo were similar, the most common
adverse events being headache and nausea. | [References]
[1] boyd m r, bacon t h, sutton d, et al. antiherpesvirus activity of 9-(4-hydroxy-3-hydroxy-methylbut-1-yl) guanine (brl 39123) in cell culture. antimicrobial agents and chemotherapy, 1987, 31(8): 1238-1242.
[2] hodge r a, perkins r m. mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl) guanine (brl 39123) against herpes simplex virus in mrc-5 cells. antimicrobial agents and chemotherapy, 1989, 33(2): 223-229. |
|
|