| Identification | More | [Name]
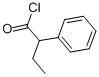
2-Phenylbutyryl chloride | [CAS]
36854-57-6 | [Synonyms]
2-ETHYL-2-PHENYLACETYL CHLORIDE
2-PHENYLBUTYRIC CHLORIDE
2-PHENYLBUTYRYL CHLORIDE
Butyryl chloride, 2-phenyl-
2-phenylbutyril chloride
2-Phenyl Butyric acid Chloride
2-Phenylbutyryl hloride
2PhenylButylrylChloride
2-Phenylbutyrylchloride,98%
2-Phenylbutanoyl chloride
a-Phenylbutyryl chloride
Benzeneacetyl chloride, a-ethyl-
2-Phenylbutanoic acid chloride | [EINECS(EC#)]
253-241-0 | [Molecular Formula]
C10H11ClO | [MDL Number]
MFCD00018811 | [Molecular Weight]
182.65 | [MOL File]
36854-57-6.mol |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S22:Do not breathe dust .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S27:Take off immediately all contaminated clothing .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3265 8/PG 2
| [WGK Germany ]
3
| [RTECS ]
ET5957500 | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
29130000 |
| Hazard Information | Back Directory | [Chemical Properties]
Clear pale yellow to yellow liquid | [Uses]
(R)-(-)-2-phenylbutyryl chloride may be employed as chiral reagent for the determination of dopamine and dopamine-derived salsolinol and norsalsolinol in human brain by GC-MS method. Chiral (S)-(+)-2-phenylbutyryl chloride may be used as derivatization reagent for the hydroxyl groups during the GC-MS assay for the enantiomers of 1,2-propanediol, 1,3-butanediol, 1,3-pentanediol and their corresponding hydroxyacids in biological fluids. | [Uses]
α-Ethyl-benzeneacetyl Chloride is used in the synthesis of mild and neutral system for selective reduction of organic functional groups. Also used in the preparation of phenothiazine derivatives for the reversible inhibition of human butyrylcholinesterase. | [General Description]
The kinetic resolution of racemic 2-phenylbutyryl chloride by sterically hindered chiral secondary alcohols has been evaluated. 2-Phenylbutyryl chloride reacts with 4-methoxybenzoyl chloride catalyzed by PdBr(Ph)(PPh3)2 to yield 1-(4-methoxyphenyl)-2-phenyl-2-buten-1-one. |
|
|