| Identification | More | [Name]
Cinacalcet hydrochloride | [CAS]
364782-34-3 | [Synonyms]
(aR)-a-Methyl-N-[3-[3-(trifluoromethyl)phenyl)propyl]-1-napthalenemethanamine Hydrochloride
(R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-�-(1-napthyl)ethylamine Hydrochloride
Cinacalcet Hydrochloride
N-[(1R)-1-naphthalen-1-ylethyl]-3-[3-(trifluoromethyl)phenyl]propan-1-amine hydrochloride
(aR)-α-Methyl-N-[3-[3-(trifluoromethyl)phenyl)propyl]-1-napthalenemethanamine Hydrochloride
N-((1R)-1-(1-Naphthyl)ethyl)-3-(3-(trifluoromethyl)phenyl)propan-1-amine hydrochloride | [EINECS(EC#)]
620-490-5 | [Molecular Formula]
C22H23ClF3N | [MDL Number]
MFCD08067750 | [Molecular Weight]
393.88 | [MOL File]
364782-34-3.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White to Tan Solid | [Melting point ]
175-177°C | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
insoluble in H2O; ≥17.85 mg/mL in DMSO; ≥56 mg/mL in EtOH | [form ]
solid | [color ]
White to off-white | [Usage]
Clinical trial in secondary hyperparathyroidism | [InChI]
InChI=1/C22H22F3N.ClH/c1-16(20-13-5-10-18-9-2-3-12-21(18)20)26-14-6-8-17-7-4-11-19(15-17)22(23,24)25;/h2-5,7,9-13,15-16,26H,6,8,14H2,1H3;1H/t16-;/s3 | [InChIKey]
QANQWUQOEJZMLL-LSQUZMQTNA-N | [SMILES]
C12C=CC=CC1=CC=CC=2[C@@H](C)NCCCC1=CC=CC(C(F)(F)F)=C1.Cl |&1:10,r| | [CAS DataBase Reference]
364782-34-3(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
Cinacalcet(364782-34-3) is the first entry in a new class of therapeutic agents called the calcimimetics.
It was launched as an oral treatment for secondary hyperparathyroidism
(SHPT) in patients with chronic kidney disease on dialysis and for
hypercalcemia in patients with parathyroid carcinoma. SHPT is associated with
increased parathyroid hormone (PTH) secretion, which is triggered by low serum
levels of calcium resulting from the failure of the kidney to clear phosphorous from
the body and its inability to produce sufficient quantities of vitamin D. The consequences
of increased PTH include stimulation of osteoclastic activity, cortical
bone resorption and marrow fibrosis. PTH secretion is primarily regulated by the
calcium-sensing receptor (CaR), which is located on the surface of the chief cell of
the parathyroid gland. Calcimimetics bind to CaR and increase the sensitivity of
CaR to extracellular calcium, thereby enabling its activation at subnormal levels of
serum calcium. As a result, in the presence of these agents, the low levels of endogenous
calcium in patients with renal failure are able to exert a suppressive effect
on PTH secretion. Parathyroid carcinoma is also associated with elevated PTH
levels, which are driven by autonomous parathyroid gland activity and subsequently
lead to hypercalcemia. Although surgical resection is the primary therapy
for treating hypercalcemia in parathyroid carcinoma patients, calcimimetics offer a
nonsurgical alternative for patients with failed parathyroidectomy, metastatic
parathyroid carcinoma, or high surgical risk. The recommended dosage of
cinacalcet for the treatment of SHPT in chronic kidney disease is 30mg once daily
at start and subsequent titration to 60, 90, 120 or 180 mg once daily. The dosage for the treatment of hypercalcemia in patients with parathyroid carcinoma is 30 mg
twice daily at start and subsequent titration to 60 or 90 mg twice daily, or 90mg
three or four times daily as necessary to normalize serum calcium level. After oral
administration of cinacalcet, maximum plasma concentration is achieved in
approximately 2 to 6 hours. It has a terminal half-life of 30 to 40 hours and
steady-state drug levels are reached within 7 days. Cinacalcet has a high volume of
distribution (1000 L) and high protein binding (93%–97%). It is extensively metabolized
in the liver, mainly by CYP3A4, CYP2D6 and CYP1A2. The primary
routes of elimination are in the urine (80%) and in the feces (15%). In Phase III
clinical trials involving 1136 patients with SHPT, administration of cinacalcet at
30–180 mg/day doses for 6 months produced 38–48% decrease in intact PTH.
Overall, 64% of patients given cinacalcet achieved at least a 30% reduction in PTH,
versus 11% of placebo patients. Calcium-phosphorous product was reduced 14%
by the active treatment and did not change in the placebo group. In a much smaller
clinical study involving 21 hypercalcemic patients with parathyroid carcinoma, administration
of 60–360 mg/day doses of cinacalcet resulted in 71% of patients
achieving a target reduction of ≥1 mg/dL in serum calcium. The most common
adverse events in these trials were nausea and vomiting. In vitro, cinacalcet is a
strong inhibitor of CYP2D6; therefore, dose adjustments may be required when
coadministered with medications that are predominantly metabolized by CYP2D6
and have a narrow therapeutic index (e.g. flecainide, vinblastine, thioridazine and
most tricyclic antidepressants). Cinacalcet is prepared in a two-step synthesis starting
from 3-[3-(trifluoromethyl)phenyl]propionaldehyde, by first condensing with
(R)-(1-naphthyl)ethylamine to form the corresponding imine and subsequent
reduction of the imine with sodium cyanoborohydride.
| [Chemical Properties]
Off-White to Tan Solid | [Originator]
NPS pharmaceuticals (US) | [Uses]
Cinacalcet hydrochloride can be used in clinical trial in secondary hyperparathyroidism.
| [Definition]
ChEBI: A hydrochloride derived from equimolar amounts of cinacalcet and hydrogen chloride. | [Brand name]
Sensipar (Amgen). | [Biological Activity]
Cinacalcet hydrochloride (364782-34-3) is a calcium-sensing receptor (CaSR) allosteric agonist. Activates CaSR signaling in cellular proliferation and phosphatidylinositol (PI) hydrolysis assays. Reduces serum parathyroid hormone levels in rats. Also potent CYP2D2 inhibitor (IC50 = 87 nM) and L-type calcium channel blocker. Orally bioavailable.
| [Mechanism of action]
Cinacalcet hydrochloride(364782-34-3) directly regulates a major regulator of parathyroid hormone (PTH) secretion, the calcium-sensitive receptor (CaR) on parathyroid master cells. Cinacalcet hydrochloride reduces circulating levels of PTH by increasing the sensitivity of the CaR to extracellular calcium.
| [Side effects]
Cinacalcet hydrochloride(364782-34-3) was generally well tolerated in clinical trials. The severity of most treatment-emergent adverse events was mild to moderate. Common side effects of Cinacalcet hydrochloride include: nausea, vomiting, or unusual tiredness. Severe may also include low calcium levels in the blood, numbness/tingling of the skin; severe muscle cramps, seizures, and abnormal heartbeat.
| [Synthesis]
General
syntheses of this class of compounds have been published, however, the specific synthesis of cinacalcet (III) has
not been available to date. The synthesis of cinacalcet, based
on a patented procedure, is depicted in Scheme 3. A mixture
of 1-acetonaphthone (21), 3-trifluoromethyl-1-propylamine
(22) and titanium (IV) isopropoxide were stirred at rt to form
the enamine intermediate which was reduced with
methanolic sodium cyanoborohydride at rt to give
corresponding racemic a-methyl amine (23). Compound 23
was resolved and then treated with HCl etherate to give
cinacalcet hydrochloride (III) as a white solid.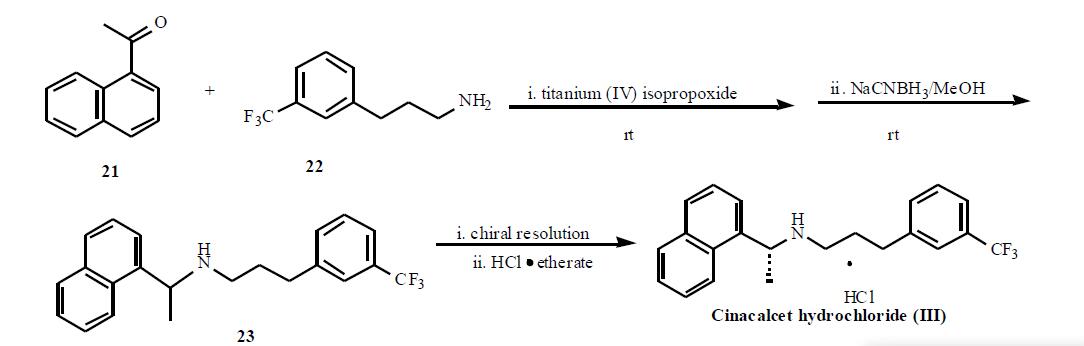
| [storage]
Store at -20°C | [References]
[1] ure?a p1, fraz?o jm. calcimimetic agents: review and perspectives. kidney int suppl. 2003 jun;(85):s91-6.
[2] colloton m1, shatzen e, wiemann b, starnes c, scully s, henley c, martin d.cinacalcet attenuates hypercalcemia observed in mice bearing either rice h-500 leydig cell or c26-dct colon tumors. eur j pharmacol. 2013 jul 15;712(1-3):8-15. |
|
|