| Identification | More | [Name]
FMOC-Ala-OH | [CAS]
35661-39-3 | [Synonyms]
9-FMOC-L-ALANINE
FMOC-ALA
FMOC-ALA-OH
FMOC-L-ALA
FMOC-L-ALANINE
FMOC-L-ALA-OH
FMOC-L-ALPHA-ALANINE
N-(9-FLUORENYLMETHOXYCARBONYL)-L-ALANINE
N-(9-FLUORENYLMETHOXYCARBONYL)-L-ALPHA-ALANINE
N-9-FLUORENYLMETHYLOXYCARBONYL-L-ALANINE
N-[(9H-FLUOREN-9-YLMETHOXY)CARBONYL]-L-ALANINE
N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-L-ALANINE
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-L-ALANINE
N-ALPHA-FMOC-L-ALA
N-ALPHA-FMOC-L-ALANINE
N-FMOC-L-ALANINE
Fmoc-DL-Alanine
L-ALANINE-2-13C, N-FMOC DERIV 99&
Fmoc-L-alanine,98%
L-Alanine-2-13C-N-FMOC | [EINECS(EC#)]
252-660-6 | [Molecular Formula]
C18H17NO4 | [MDL Number]
MFCD00037139 | [Molecular Weight]
311.33 | [MOL File]
35661-39-3.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powde | [Melting point ]
147-153 °C (lit.) | [alpha ]
-19 º (c=1,DMF) | [Boiling point ]
451.38°C (rough estimate) | [density ]
1.2626 (rough estimate) | [refractive index ]
-18.5 ° (C=1, DMF) | [storage temp. ]
2-8°C
| [solubility ]
DMSO (Slightly), DMF (Sparingly), Methanol (Slightly) | [form ]
Solid | [pka]
3.91±0.10(Predicted) | [color ]
White | [optical activity]
[α]20/D 18°, c = 1 in DMF | [Water Solubility ]
Soluble in water. | [Detection Methods]
T,NMR,HPLC,Rotation | [BRN ]
2225975 | [InChIKey]
QWXZOFZKSQXPDC-NSHDSACASA-N | [CAS DataBase Reference]
35661-39-3(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S27:Take off immediately all contaminated clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [HazardClass ]
IRRITANT | [HS Code ]
29242990 |
| Hazard Information | Back Directory | [Chemical Properties]
white to light yellow crystal powde | [Uses]
N-Fmoc-L-alanine is potentially useful for proteomics studies and solid phase peptide synthesis techniques. Alanine is one of the simplest amino acids - a methyl group as the side chain. This small side chain confers a high degree of flexibility when incorporated into a polypeptide chain. The Fmoc group is typically removed with a base such as pyridine - an orthogonal de-protection strategy to the acid labilie Boc group. | [Preparation]
A solution of the Fmoc-OX derivatives (20 mmol) in 100 mL acetone was added dropwise to a stirred solution of Alanine (20 mmol) and NaHCO3 (50 mmol) in 100 mL water. After stirring overnight the reaction mixture was concentrated under reduced pressure and then extracted with CH2Cl2 (50 mL) to remove the unreacted Fmoc-OX derivatives. The reaction mixture was cooled and acidified with 10% HCl to congo red litmus paper to give a white solid, which was filtered and washed with water several times, dried to give a white solid (FMOC-Ala-OH).Yield=89.5%.
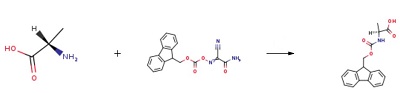 | [reaction suitability]
reaction type: Fmoc solid-phase peptide synthesis |
|
|