| Identification | More | [Name]
2,2'-Bis(trifluoromethyl)benzidine | [CAS]
341-58-2 | [Synonyms]
2,2'-BIS-(TRIFLUORMETHYL) BENZIDINE
2,2'-BIS(TRIFLUOROMETHYL)BENZIDINE
2,2'-DI(TRIFLUOROMETHYL)BENZIDINE
22TFMB
4,4'-DIAMINO-2,2'-BIS(TRIFLUOROMETHYL)BIPHENYL
ABL-21
2,2'-Bis(trifluoromethyl)-4,4'-diamino biphenyl
2,2'-Bis(trifluoromethyl)benzidine 97%
2,2'-Bis(trifluoromethyl)benzidine97%
2,2'-Bis(trifluoromethyl)-4,4'-biphenyldiamine | [EINECS(EC#)]
671-105-2 | [Molecular Formula]
C14H10F6N2 | [MDL Number]
MFCD00190155 | [Molecular Weight]
320.23 | [MOL File]
341-58-2.mol |
| Chemical Properties | Back Directory | [Melting point ]
183 °C | [Boiling point ]
376.9±42.0 °C(Predicted) | [density ]
1.415±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,2-8°C | [solubility ]
soluble in Methanol | [form ]
powder to crystal | [pka]
3.23±0.10(Predicted) | [color ]
White to Light yellow to Light orange | [InChI]
InChI=1S/C14H10F6N2/c15-13(16,17)11-5-7(21)1-3-9(11)10-4-2-8(22)6-12(10)14(18,19)20/h1-6H,21-22H2 | [InChIKey]
NVKGJHAQGWCWDI-UHFFFAOYSA-N | [SMILES]
C1(C2=CC=C(N)C=C2C(F)(F)F)=CC=C(N)C=C1C(F)(F)F | [CAS DataBase Reference]
341-58-2(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
T,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R45:May cause cancer.
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment . | [Safety Statements ]
S22:Do not breathe dust .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
2811 | [Hazard Note ]
Toxic | [HazardClass ]
IRRITANT-HARMFUL | [HS Code ]
29215900 |
| Hazard Information | Back Directory | [Description]
2,2'-Bis(trifluoromethyl)benzidine (TFMB,341-58-2) is a fluorinated benzidine derivative with two trifluoromethyl substituents at the 2- and 2'-positions. 2,2'-Bis(trifluoromethyl)benzidine (TFMB) is a rigid and aromatic polyimide used in the synthesis of metal-organic frameworks (MOFs) and covalent organic frameworks (COFs) of high specific surface area.TFMB is also synthesised as UV-resistant and colourless polyimide thin films for optoelectronic applications. TFMB is also used to synthesise UV resistant and colourless polyimide films for optoelectronic applications. The two fluoromethyl groups have a strong electron-withdrawal effect, which reduces the electron dispersion in the π-orbitals of the fully conjugated polymer backbone. This effect results in a much higher transparency of the polyimide film compared to polyimide films containing methyl substituents. This polyimide can be used for the encapsulation of solar cells with MOFs cluster light diffusers.
| [Uses]
2,2'-Bis(trifluoromethyl)benzidine is used for producing a high strength flexible transparent polyimide material.
| [Synthesis]
This 2,2′-bis(trifluoromethyl)-4,4′-dinitrobiphenyl crystal of 42.5 g, toluene of 127.5 g and 5% Pd/C (AER-TYPE: 50% water contained product) of 1.8 g manufactured by N.E. CHEMCAT Corp. were fed in an autoclave made of stainless steel, after the system was replaced sufficiently with hydrogen, pressured with hydrogen for a reaction pressure to be 1 MPa and reaction was carried out at a reaction temperate of 60 °C for 6 hours. After reaction, catalyst was filtered away, toluene in this reaction liquid was distilled away under reduced pressure, concentrated and crystallized, 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl of 28.5 g (purity converted) was obtained by solid-liquid separation.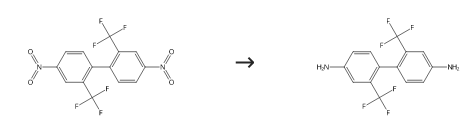 |
|
|