| Identification | More | [Name]
5-Azacytidine | [CAS]
320-67-2 | [Synonyms]
4-AMINO-1-B-D-RIBOFURANOSYL-SYM-TRIAZINE-2(1H)-ONE
4-AMINO-1-(BETA-D-RIBOFURANOSYL)-1,3,5-TRIAZIN-2(1H)-ONE
4-AMINO-1-BETA-D-RIBOFURANOSYL-1,3,5-TRIAZINE-2[1H]-ONE
4-AMINO-1-BETA-D-RIBOFURANOSYL-5-TRIAZIN-2[1H]-ONE
4-AMINO-1-BETA-D-RIBOFURANOSYL-S-TRIAZIN-2[1H]ONE
5-AZAC
5-AZACYTIDINE
AZACITIDINE
AZACYTIDINE
LADAKAMYCIN
4-amino-1-beta-d-ribofuranosyl-s-triazin-2(1h)-on
5-triazin-2(1h)-one,4-amino-1-beta-d-ribofuranosyl-3
antibioticu18496
mylosar
nci-c01569
nsc-102816
u18496
4-Amino-1-(β-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one
5 AZC
5-AC | [EINECS(EC#)]
206-280-2 | [Molecular Formula]
C8H12N4O5 | [MDL Number]
MFCD00006539 | [Molecular Weight]
244.2 | [MOL File]
320-67-2.mol |
| Chemical Properties | Back Directory | [Appearance]
White-to-Off-White Crystalline Solid | [Melting point ]
226-232 °C (dec.)(lit.)
| [alpha ]
40 º (C=1, H2O 22 ºC) | [Boiling point ]
387.12°C (rough estimate) | [density ]
1.4287 (rough estimate) | [refractive index ]
1.6590 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
Soluble in DMSO (up to 25 mg/ml), or in Water (up to 12 mg/ml). | [form ]
lyophilized powder
| [pka]
13.46±0.70(Predicted) | [color ]
White to Off-white | [biological source]
synthetic (organic) | [Water Solubility ]
0.5-1.0 g/100 mL at 21 ºC | [Usage]
A potent growth inhibitor and cytotoxic agent. It acts as a demethylating agent by inhibiting DNA methyltransferase | [Merck ]
887 | [BRN ]
620461 | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20° for up to 1 month. | [InChIKey]
NMUSYJAQQFHJEW-WGDKFINWSA-N | [LogP]
-2.191 (est) | [CAS DataBase Reference]
320-67-2(CAS DataBase Reference) | [IARC]
2A (Vol. 50) 1990 | [Storage Precautions]
Light sensitive | [EPA Substance Registry System]
320-67-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R45:May cause cancer.
R22:Harmful if swallowed. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S22:Do not breathe dust .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [WGK Germany ]
3
| [RTECS ]
XZ3017500
| [F ]
10-23 | [HS Code ]
29349900 | [Safety Profile]
Confirmed carcinogen
with experimental carcinogenic,
neoplastigenic, tumorigenic data. Poison by
ingestion, intravenous, and intraperitoneal
routes. Human systemic effects by
intravenous route: nausea, vomiting and
dlarrhea, reduction in white cell count
(luekopenia and agranulocytosis). An
experimental teratogen. Other experimental
reproductive effects. Human mutation data
reported. A skin irritant. When heated to
decomposition it emits toxic fumes of NOx. | [Hazardous Substances Data]
320-67-2(Hazardous Substances Data) | [Toxicity]
LD50 in mice (mg/kg): 115.9 i.p.; 572.3 orally (Palm, Kensler) |
| Hazard Information | Back Directory | [General Description]
White crystalline powder. | [Reactivity Profile]
5-AZACYTIDINE(320-67-2) is sensitive to light (may discolor). 5-AZACYTIDINE(320-67-2) is sensitive to oxidation. 5-AZACYTIDINE(320-67-2) is unstable in solution. 5-AZACYTIDINE(320-67-2) undergoes hydrolysis in aqueous buffers. This chemical is incompatible with strong oxidizers. | [Air & Water Reactions]
Slightly water soluble. Unstable in solution. | [Potential Exposure]
A growth inhibitor (DNA methyltransferase
inhibitor). A cytotoxic agent and chemotherapeutic
agent used to treat angina pectoris, an ischemic heart disease
symptom. Occupational exposure to azacitidine could
occur among health professionals and support staff (including
custodians) by dermal contact, inhalation, or accidental
ingestion during drug preparation or administration or
cleanup of medical waste, including disposal of excretions
from treated patients (Zimmerman et al. 1981, NIOSH
2004). The National Occupational Exposure Survey (conducted
from 1981 to 1983) estimated that 1069 healthservices
workers, including 698 women, potentially
were exposed to azacitidine. Azacitidine may be
produced synthetically or isolated from the bacterium
Streptoverticillium ladakanus .
Incompatibilities: Azacitidine is Incompatible with oxidizers
(chlorates, nitrates, peroxides, permanganates, perchlorates,
chlorine, bromine, fluorine, etc.); contact may cause
fires or explosions. Keep away from alkaline materials,
strong bases, strong acids, oxoacids, epoxides. Contact with
alkali metals, nitrides, and strong reducing agents such as
hydrides may form flammable and/or toxic gases. May react
with anhydrides forming acids and esters, generating noticeable
heat, and also with oxoacids and carboxylic acids to
form esters plus water, but the heat of reaction in the latter
case typically is low. Keep away from isocyanates and
epoxides; may initiate their polymerization. Azacitidine is
sensitive to light and oxidation and unstable in solution. It
undergoes hydrolysis in aqueous buffers. | [Fire Hazard]
Flash point data for this chemical are not available; however, 5-AZACYTIDINE is probably combustible. | [First aid]
Eyes: First check the victim for contact lenses
and remove if present. Flush victim’s eyes with water or
normal saline solution for 20 to 30 minutes while simultaneously
calling a hospital or poison control center. Do not
put any ointments, oils, or medication in the victim’s eyes
without specific instructions from a physician. Immediately
transport the victim after flushing eyes to a hospital even if
no symptoms (such as redness or irritation) develop. Skin:
Immediately flood affected skin with water while removing
and isolating all contaminated clothing. Gently wash all
affected skin areas thoroughly with soap and water. If
symptoms such as redness or irritation develop, immediately
call a physician and be prepared to transport the
victim to a hospital for treatment. Inhalation: Immediately
leave the contaminated area; take deep breaths of fresh air.
IMMEDIATELY call a physician and be prepared to transport
the victim to a hospital even if no symptoms (such as
wheezing, coughing, shortness of breath, or burning in the
mouth, throat, or chest) develop. Provide proper respiratory
protection to rescuers entering an unknown atmosphere.
Whenever possible, SCBA (SCBA) should be used; if not
available, use a level of protection greater than or equal to
that advised under Protective Clothing. INGESTION: Do
not induce vomiting. If the victim is conscious and not convulsing,
give 1 or 2 glasses of water to dilute the chemical
and immediately call a hospital or poison control center. Be
prepared to transport the victim to a hospital if advised by
a physician. If the victim is convulsing or unconscious, do
not give anything by mouth, ensure that the victim’s airway
is open and lay the victim on his/her side with the head
lower than the body. Do not induce vomiting. Immediately
transport the victim to a hospital. | [Shipping]
UN3249 Medicine, solid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials. | [Incompatibilities]
Azacitidine is Incompatible with oxidizers
(chlorates, nitrates, peroxides, permanganates, perchlorates,
chlorine, bromine, fluorine, etc.); contact may cause
fires or explosions. Keep away from alkaline materials,
strong bases, strong acids, oxoacids, epoxides. Contact with
alkali metals, nitrides, and strong reducing agents such as
hydrides may form flammable and/or toxic gases. May react
with anhydrides forming acids and esters, generating noticeable
heat, and also with oxoacids and carboxylic acids to
form esters plus water, but the heat of reaction in the latter
case typically is low. Keep away from isocyanates and
epoxides; may initiate their polymerization. Azacitidine is
sensitive to light and oxidation and unstable in solution. It
undergoes hydrolysis in aqueous buffers. | [Chemical Properties]
White crystalline solid or powder. | [Chemical Properties]
White-to-Off-White Crystalline Solid | [Waste Disposal]
It is inappropriate and possibly
dangerous to the environment to dispose of expired or
waste drugs and pharmaceuticals by flushing them down
the toilet or discarding them to the trash. Household quantities
of expired or waste pharmaceuticals may be mixed
with wet cat litter or coffee grounds, double-bagged in
plastic, discard in trash. Larger quantities shall carefully
take into consideration applicable DEA, EPA, and FDA
regulations. If possible return the pharmaceutical to the
manufacturer for proper disposal being careful to properly
label and securely package the material. Alternatively, the
waste pharmaceutical shall be labeled, securely packaged
and transported by a state licensed medical waste contractor
to dispose by burial in a licensed hazardous or toxic waste
landfill or incinerator. | [Originator]
Pharmion (US) | [Uses]
A potent growth inhibitor and cytotoxic agent. It acts as a demethylating agent by inhibiting DNA methyltransferase | [Uses]
antianginal | [Uses]
Antineoplastic;'Antimetabolite | [Definition]
ChEBI: A N-glycosyl-1,3,5-triazine that is 4-amino-1,3,5-triazin-2(1H)-one substituted by a beta-D-ribofuranosyl residue via a N-glycosidic linkage. | [Manufacturing Process]
A mixture of 1-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)-4-methylthio-1,2-
dihydro-1,3,5-triazin-2-one (0.5875 g), absolute methanol (5 ml) and a
normal methanolic sodium methoxide solution (1.2 ml) is stirred at room
temperature with the exclusion of atmospheric moisture (a guard tube filled
with potassium hydroxide pellets is fitted to the reaction vessel). The starting
compound passes into solution in the course of 5 min. The resulting solution is
allowed to stand at room temperature for 45 min and then the cations are
removed by passage of the solution through a column packed with 10 ml of a
weakly acidic cation exchange resin in the H+ form prewashed with water and
methanol. The methanolic effluent (60 ml) is evaporated under reduced
pressure at 30°C, the residue is dissolved in methanol (20 ml) and the
solution once again is evaporated and the 1-β-D-ribofuranosyl-4-methoxy-1,2-
dihydro-1,3,5-triazin-2-one was obtained.
The residual crude crystalline 1β-D-ribofuranosyl-4-methoxy-1,2-dihydro-
1,3,5-triazin-2-one is dissolved in a 10% solution of dry ammonia in absolute
methanol (4 ml) and the whole reaction mixture is allowed to stand in a
stoppered flask for 30 min at room temperature (the product begins to
deposit in the course of 5 min) and for 12 h in a refrigerator at -10°C. The
resulting 5-azacytidine is collected with suction, washed with methanol and
dried under reduced pressure. A yield of 0.216 g (88.6%) of 5-azacytidine,
that is [1-β-D-ribofuranosyl-4-amino-1,3,5-triazin-2(1H)-one], melting point
232°-234°C (dec.), is obtained. | [Brand name]
Vidaza (Pharmion). | [Therapeutic Function]
Antineoplastic | [Biological Functions]
Azacitidine is given subcutaneously for the treatment of myelodysplastic syndrome, and serum levels generally are maximized within 30 minutes. The parent drug and its metabolites are excreted in the urine. Azacitidine is carcinogenic and teratogenic in rodents, and leukopenia, thrombocytopenia, and neutropenia are the most common reasons for drug discontinuation or dosage reduction. | [Biological Activity]
DNA methyltransferase inhibitor. Incorporates into DNA forming covalent adducts with cellular DNMT1, depleting enzyme activity. Induces demethylation and reactivation of silenced genes. Improves the efficiency of reprogramming of stem cells. | [Biochem/physiol Actions]
5-Azacytidine is a deoxycytidine analog and a demethylating agent. It acts as a potential antineoplastic agent for acute myelogenous leukemia. 5-Azacytidine activates repressed genes by inhibiting DNA methylation. 5-Azacytidine also affects protein synthesis by altering the RNA function and stability. | [Clinical Use]
Antineoplastic agent:
Treatment of people not eligible for stem cell
transplants with myelodysplastic syndromes,
chronic myelomonocytic leukaemia or acute myeloid
leukaemia | [Synthesis]
The triazine ring of azacitidine is sensitive to water; this characteristic has
made the synthesis of azacitidine a challenge, especially in
manufacturing at commercial scale. A number of reports have
appeared in order to avoid the use of water; however, these
methods all have additional problems that render them
undesirable for the large scale synthesis. A recent
improved synthesis is depicted in the Scheme. 5-
Azacytosine (1) was bis-silylated with HMDS in the
presence of (NH4)SO4 to furnish trimethylsilylated
azacytosine (2) in greater than 90% yield. Coupling of
silylated azacytosine 2 with 1,2,3,5-tetra-O-acetyl-b-Dribofuranose
(3) in DCM in the presence of TMS-triflate
provided protected 5-azacitidine 4. The acetyl groups were
then removed by using NaOMe in MeOH at rt. The crude
azacitidine was crystallized from DMSO/MeOH to provide
pure azacitidine (I).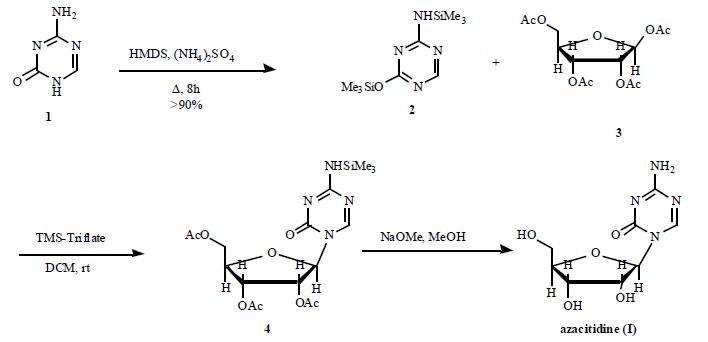
| [Drug interactions]
Potentially hazardous interactions with other drugs
None known | [Carcinogenicity]
Azacitidine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. | [Metabolism]
Azacitidine undergoes spontaneous hydrolysis and
deamination mediated by cytidine deaminase.
Following IV administration of radioactive azacitidine to
5 cancer patients, the cumulative urinary excretion was
85% of the radioactive dose. Faecal excretion accounted
for <1% of administered radioactivity over three days.
Mean excretion of radioactivity in urine following SC
administration of [14C]-azacitidine was 50%. | [storage]
Room temperature | [References]
1) Giraldo et al. (2011), Inhibition of DNA Methylation in somatic cells ; Methods Mol. Biol., 791 145
2) Brueckner et al. (2005), Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA Methyltransferases; Cancer Res., 65 6305
3) Mikkelsen et al. (2008), Dissecting direct reprogramming through integrative genomic analysis; Nature, 454 49
4) Qian et al. (2011), 5-Azacytidine induces cardiac differentiation of human umbilical cord-derived mesenchymal stem cells by activating extra cellular regulated kinase; Stem Cells Dev., 21 67
5) Kiziltepe et al. (2007), 5-Azacytidine, a DNA Methyltranserase inhibitor, induces ATR-mediated DNA double-strand break responses, apoptosis, and synergistic cytotoxicity with doxorubicin and bortezomib against multiple myeloma cells; Mol. Cancer Ther., 6 1718 |
| Questions And Answer | Back Directory | [Description]
Azacytidine also referred to as Azacytidine and 5-azacytidine (brand name: Vidaza) is an anti-cancer chemotherapy medication. The drug is classified as demethylation and antimetabolite agent. Azacytidine acts by inhibiting the growth and proliferation of cancer cells in the body. The drug is indicated for the treatment of specific types of blood cell disorders and bone marrow cancers.
| [Indication]
Azacytidine is prescribed for treating patients with secondary types of myelodysplastic syndrome, including refractory anemia that presents in the form of ringed sideroblasts (especially if it necessitates transfusion), or it presents itself alongside thrombocytopenia or neutropenia. Also, it is used in treating refractory anemia accompanied by excess blasts, refractory anemia characterized by excess blasts in transition (currently classified as chronic myelogenous leukemia that is accompanied by multilineage dysplasia), and acute myelomonocytic leukemia.
| [Contraindications]
Azacytidine is contraindicated in patients who have acute malignant hepatic tumors or known hypersensitivity to the drug or mannitol.
| [Dosage]
For treatment of patients with myelodysplastic syndrome, the initial dose is 75mg/m2 administered intravenously or subcutaneously in daily doses for 7 days in 4-week intervals. The recommended maintenance dose may be increased to 100mg/m2 if there are no noteworthy effects after 2 treatment cycles and if the patient does not experience additional toxicity other than vomiting and nausea.
Patients should undergo treatment that lasts for a minimum of 4 cycles. However, a partial or complete response may necessitate additional cycles other than the recommended 4 cycles. Treatment should not be discontinued if the patient is experiencing positive outcomes from the therapy.
| [Mechanism of Action]
5-Azacytidine is a chemical analog that is closely linked with the cytosine nucleoside in ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Azacytidine influences antineoplastic activity through two main mechanisms; suppression of DNA methyltransferase when administered in low doses, which results in hypomethylation of DNA, and unmediated cytotoxicity in anomalous hematopoietic cells present in the bone marrow by its integration into RNA and DNA at high doses which causes the death of the cells.
Since Azacytidine is considered a ribonucleoside, it integrates itself into RNA to a greater extent than DNA. The integration into RNA results in the dissimulation of malfunctioning methylation, polyribosomes, and recipient function of replicated RNA, and suppression of protein production.
The integration of Azacytidine into DNA results in a covalent bond that is characterized by DNA methyltransferases, which inhibits DNA synthesis and resultant cytotoxicity.
| [Elimination]
Intravenous administration of the radioactive form of the drug to cancer patients results in 85% elimination of Azacytidine through urinary excretion. Fecal excretion of the radioactive dose takes place in <1% of the administered drug in 3 days. The mean elimination of radioactivity through urine accounts for 50% of 14C-azacytidine administration.
| [Adverse reactions]
Common side effects associated with Azacytidine in more than 30% of the patients include low white blood cell count, fever, vomiting, low platelet count, anemia, and nausea. At nadir, 10-17days of chemotherapy cycles, one may also experience petechiae, ecchymosis, constipation, redness at the injection site and fatigue.
Other side effects associated with Azacytidine in about 10-29% of the patients may include insomnia, depression, itching, upper respiratory infection, hypokalemia, anxiety, skin rash, abdominal pain, weight loss, nosebleed, chest pain, swelling on the ankles, dizziness, confusion, back pain, sore throat, poor appetite, headache, myalgia and arthralgia, pain at the injection site, chills, weakness, shortness of breath and coughs.
It is important to contact health care provider if one is experiencing diarrhea (4-6 episodes in 24 hours). A patient should also contact the doctor in case he/she has nausea that interferes with their ability to eat. If one has bloodstained urine, constipation that persists regardless of laxative use, extreme fatigue, tarry or bloodstained stools, vomiting (4-5 episodes in a span of 24 hours), signs of infection such as productive coughs or painful urination, and inability to eat or take fluids for more than 24 hours.
| [Precautions]
A patient should notify their healthcare provider if they are taking any other medications which may include herbal remedies, vitamins, and over-the-counter medications before receiving an Azacytidine prescription. A patient should not receive any vaccination or immunization while they are on Azacytidine treatment.
Breastfeeding is not recommended while one is taking this drug. A patient should also inform their healthcare provider if they are pregnant or intending to get pregnant before starting Azacytidine treatment.
Azacytidine may cause neutropenia, anemia, and thrombocytopenia. Since the drug may result in hepatotoxicity amongst patients with acute hepatic impairment, caution should be taken during the administration of Azacytidine in patients with liver disease.
Renal toxicity that may range from renal failure to increased serum creatinine and death has been reported amongst patients who have been accorded intravenous treatment with Azacytidine in combination treatment with other chemotherapeutic medications for nonMDS cases.
The drug may also result in acute tumor lysis syndrome for patients with MDS. Azacytidine poses a threat to a developing embryo/fetus based on its mechanism of action.
|
|
|