| Identification | More | [Name]
Zidovudine | [CAS]
30516-87-1 | [Synonyms]
1-((2R,4S,5S)-4-AZIDO-5-HYDROXYMETHYL-TETRAHYDRO-FURAN-2-YL)-5-METHYL-1H-PYRIMIDINE-2,4-DIONE
1-(4-azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1h-pyrimidine-2,4-dione
3'-AZIDO-3'-DEOXYTHYMIDINE
AZIDOTHYMIDINE
AZT
ZIDOVUDINE
3’-azido-3’-deoxy-thymidin
bwa509u
bw-a509u
retrovir
ZIDOVUDINE. ANTI-VIRAL
Zidovudine(AZT)
ZIDOVUDINE EP III
3'-AZIDO-3'-DEOXYTHYMIDINE (AZIDOTHYMIDI NE, AZT)
ZIDOVUDINE (AZIDOTHYMIDINE,AZT)
Azt(Zidovudine)
ZidovudineUsp28
ZidovudineB20081(Azt)
Zidovudine EPIII/USD24
Azitidine | [EINECS(EC#)]
623-849-4 | [Molecular Formula]
C10H13N5O5 | [MDL Number]
MFCD00006536 | [Molecular Weight]
283.24 | [MOL File]
30516-87-1.mol |
| Chemical Properties | Back Directory | [Appearance]
Off White Crystalline Powder | [Melting point ]
113-115 °C (lit.) | [alpha ]
D25 +99° (c = 0.5 in water) | [Boiling point ]
410.43°C (rough estimate) | [density ]
1.3382 (rough estimate) | [refractive index ]
47 ° (C=1, H2O) | [Fp ]
9℃ | [storage temp. ]
−20°C
| [solubility ]
H2O: 50 mg/mL
| [form ]
Powder | [pka]
pKa 9.53(H2O
t = 25.0±0.1
I = 0.00) (Uncertain) | [color ]
White to Off-white | [Water Solubility ]
1-5 g/100 mL at 17 ºC | [Sensitive ]
Light Sensitive & Hygroscopic | [Usage]
A potent and selective inhibitor of HIV-1 replication | [Merck ]
10123 | [BRN ]
3595791 | [BCS Class]
1,3 | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. | [InChIKey]
HBOMLICNUCNMMY-BWZBUEFSSA-N | [CAS DataBase Reference]
30516-87-1(CAS DataBase Reference) | [IARC]
2B (Vol. 76) 2000 | [EPA Substance Registry System]
30516-87-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R40:Limited evidence of a carcinogenic effect.
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
UN1230 - class 3 - PG 2 - Methanol, solution | [WGK Germany ]
3
| [RTECS ]
XP2072000
| [F ]
10 | [Hazard Note ]
Harmful | [HS Code ]
29349900 | [Hazardous Substances Data]
30516-87-1(Hazardous Substances Data) | [Toxicity]
LD50 in male, female mice, male, female rats (mg/kg): 3568, 3062, 3084, 3683 orally; >750 i.v. (all species) (Ayers) |
| Hazard Information | Back Directory | [General Description]
Slightly off-white odorless powdery solid. | [Reactivity Profile]
3'-AZIDO-3'-DEOXYTHYMIDINE(30516-87-1) is a azido compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. | [Air & Water Reactions]
Dust may form an explosive mixture in air. Water soluble. Hydrolysis occurs in strongly basic solutions . | [Fire Hazard]
Flash point data for this chemical are not available; however, 3'-AZIDO-3'-DEOXYTHYMIDINE is probably combustible. | [Description]
Zidovudine, also known as azidothymidine (AZT), is an antiviral agent acting via reverse
transcnptase inhibition. It was first launched in the U.K. and subsequently introduced in
over a dozen countries for the management of severe manifestations of HIV infection. In
patients with AIDS and ARC, zidovudine reduces the risk of opportunistic infections and
prolongs survival time. In symptom-free patients it shows promise in halting further
immunological deterioration. | [Chemical Properties]
Off White Crystalline Powder | [Originator]
Detroit Inst. Cancer Res. (USA) | [Uses]
A potent and selective inhibitor of HIV-1 replication | [Uses]
antibacterial | [Uses]
Zidovudine is an antiretroviral drug that is clinically active against HIV-1 and is intended
to treat HIV-infected patients. Zidovudine is an analog of thymidine that inhibits replica�tion of the AIDS virus. It also turned into mono-, di-, and triphosphates by the same cel�lular enzymes that catalyze phosphorylation of thymidine and thymidine nucleosides.
Zidovudine-triphosphate is then included in the terminal fragment of the growing chain of
viral DNA by viral reverse transcriptase, thus causing the viral DNA chain to break apart
in cells infected with the virus.
Zidovudine has been authorized for treating patients with AIDS. It significantly pro�longs the life of the patient, although it has a number of toxic effects. Synonyms of this
drug are azidothymidine and retrovir. | [Definition]
ChEBI: A pyrimidine 2',3'-dideoxyribonucleoside compound having a 3'-azido substituent and thymine as the nucleobase. | [Indications]
Zidovudine was the first agent to be used to prevent the
transmission of HIV from a pregnant woman to her
child. It was given to the mother at 14 to 34 weeks’ gestation
and to the child for the first 6 weeks of life.
Current combination therapies employ zidovudine with
another NRTI and a protease inhibitor. | [Manufacturing Process]
Preparation of 2,3'-anhydrothymidine
Thymidine (85.4 g; 0.353 mol) was dissolved in 500 mL dry DMF (dimethyl
formamide) and added to N-(2-chloro-1,1,2-trifluoroethyl)diethylamine (100.3
g; 0.529 mol) [prepared according to the method of D. E. Ayer, J. Med. Chem.
6, 608 (1963)]. This solution was heated at 70°C for 30 minutes then poured
into 950 mL ethanol with vigorous stirring. The product precipitated from this
solution and was filtered. The ethanol supernatant was refrigerated then
filtered to yield a total of 47.75 g (0.213 mol; 60.3%) of 2,3'-
anhydrothymidine; melting point 228°-230°C.
Preparation for 3'-azido-3'-deoxythymidine
2,3'-Anhydrothymidine (25 g; 0.1115 mol) and NaN 3 (29 g; 0.446 mol) was
suspended in a mixture of 250 mL DMF and 38 mL H 2 O. The reaction was
refluxed for 5 hours at which time it was poured into 1 liter of H 2 O. This
aqueous solution was extracted with ethyl acetate (EtOAc) (3x700 ml). The
EtOAc was dried over Na 2 SO 4 , filtered, and then EtOAc was removed in vacuo
to yield a viscous oil. This oil was stirred with 200 mL water resulting in a
solid, 3'-azido-3'-deoxythymidine, 9.15 g (0.0342 mol); 30.7%; melting point
116°-118°C.
| [Brand name]
Retrovir (GlaxoSmithKline). | [Therapeutic Function]
Antiviral, Antineoplastic | [Antimicrobial activity]
Zidovudine is active against HIV-1, HIV-2 and HTLV-1. | [Acquired resistance]
As with stavudine, mutations at position 41, 67 and 70, and
positions 210, 215 and 219 (the ‘thymidine analog mutations’)
of the reverse transcriptase genes are associated with
diminished antiretroviral efficacy. | [Pharmaceutical Applications]
An analog of thymidine formulated for oral or intravenous use. | [Biochem/physiol Actions]
Reverse transcriptase inhibitor active against HIV-1 virus. | [Mechanism of action]
Zidovudine (AZT , ZDV) is an analogue of thymidine in which the azido group is substituted at the 3-carbon atom of the dideoxyribose moiety. It is active against RNA tumor viruses (retroviruses) that are the causative agents of AIDS and T-cell leukemia. Retroviruses, by virtue of RT, direct the synthesis of a provirus (DNA copy of a viral RNA genome). Proviral DNA integrates into the normal cell DNA, leading to the HIV infection. Zidovudine is converted to 5′-mono-, di-, and triphosphates by the cellular thymidine kinase. These phosphates are then incorporated into proviral DNA, because RT uses ZDV-triphosphate as a substrate. This process prevents normal 5′,3′-phosphodiester bonding, resulting in termination of DNA chain elongation because of the presence of an azido group in ZDV. The multiplication of HIV is halted by selective inhibition of RT and, thus, viral DNA polymerase by ZDV-triphosphate at the required dose concentration. Zidovudine is a potent inhibitor of HIV-1, but it also inhibits HIV-2 and EBV. | [Pharmacokinetics]
Oral absorption: 65%
Cmax 300 mg twice daily: 2.3 mg/L
Plasma half-life: 1.1 h
Volume of distribution: 1.6 L/kg
Plasma protein binding; 34–38%
Absorption and distribution
It is absorbed rapidly and almost completely following oral administration. Absorption is not significantly affected by food. It appears to undergo widespread body distribution. CNS penetration is fairly good. The semen:plasma ratio varies from 0.95 to 13.5 (mean 5.9). It is secreted into breast milk.
Metabolism and excretion
Following hepatic metabolism (glucuronidation), elimination is primarily renal. After oral administration, urinary recovery of zidovudine and its glucuronide metabolite accounted for 14% and 74% respectively of the dose, with a total urinary recovery of 90%.
In severe renal impairment, clearance was about half that reported in subjects with normal renal function Accumulation may occur in patients with hepatic impairment due to decreased glucuronidation. | [Clinical Use]
Treatment of HIV infection in adults and children (in combination with
other antiretroviral drugs)
Reduction of maternal transmission of HIV to the fetus | [Side effects]
In common with other drugs in this class, use has been associated
with episodes of fatal and non-fatal lactic acidosis
and hepatomegaly with steatosis. Careful clinical evaluation
is needed in patients with evidence of hepatic abnormality.
Myelosuppression may occur within the first 4–6 weeks of
therapy. Hematological parameters should be monitored during
this period, with prompt dose modification or switch if
abnormalities are observed. Treatment with reduced doses
may be attempted in some patients once bone marrow recovery
has been observed. Myopathy is rarely seen with the use
of the current dosing regimens.
Co-administration with drugs known to cause nephrotoxicity,
cytotoxicity or which interfere with red or white blood
cell number and function may increase the risk of toxicity.
Probenecid and trimethoprim may reduce renal clearance
of zidovudine, and other drugs that are metabolized by
glucuronidation may interfere with its metabolism. | [Safety Profile]
Moderately toxic by intravenousroute. Human systemic effects by ingestion: aplasticanemia, changes in blood cell count, convulsions or effect on seizure threshold, headache, nails, retinal changes.Human mutation data reported. | [Synthesis]
Zidovudine is 3�-azido-3�-deoxytimidine (36.1.26), is synthesized from
1-(2�-deoxy-5�-O-trityl-|?-D-lyxosyl)thymine, which is treated with methansulfonyl chlo�ride in pyridine to make the corresponding mesylate 36.1.24. Replacing the methyl group
with an azide group using lithium azide in dimethylformamaide makes the product 36.1.25
with inverted configuration at C3 of the furanosyl ring. Heating this in 80% acetic acid
removes the trityl protection, giving zidovudine.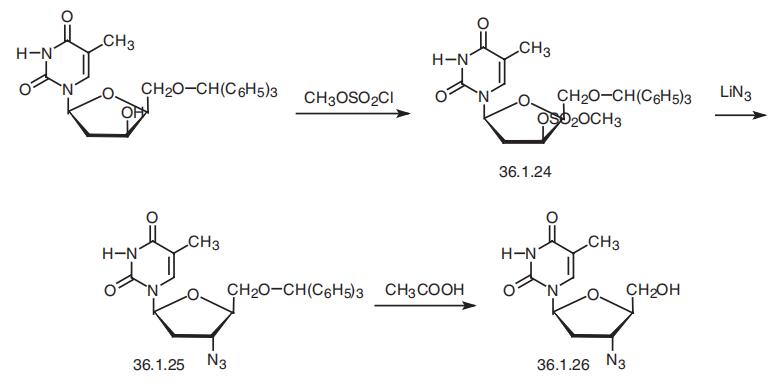
| [Veterinary Drugs and Treatments]
In veterinary medicine, zidovudine may be useful for treating feline
immunodeficiency virus (FIV) or feline leukemia virus (FeLV).
While zidovudine can reduce the viral load in infected cats and improve
clinical signs, it may not alter the natural course of the disease
to a great extent. | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: absorption reduced by clarithromycin;
avoid concomitant use with rifampicin.
Antiepileptics: phenytoin levels may be raised
or lowered; concentration possibly increased by
valproate (increased risk of toxicity).
Antifungals: concentration increased by fluconazole.
Antivirals: profound myelosuppression with
ganciclovir and valganciclovir - avoid if possible;
increased risk of granulocytopenia with nevirapine;
increased risk of anaemia with ribavirin - avoid;
effects of stavudine inhibited - avoid concomitant
use; concentration reduced by tipranavir.
Orlistat: absorption possibly reduced by orlistat.
Probenecid: excretion reduced by probenecid,
increased risk of toxicity. | [Metabolism]
Zidovudine is metabolised intracellularly to the antiviral
triphosphate. It is also metabolised in the liver, mainly to
the inactive glucuronide, and is excreted in the urine as
unchanged drug and metabolite.
The 5'-glucuronide of zidovudine is the major metabolite
in both plasma and urine, accounting for approximately
50-80
% of the administered dose eliminated by renal
excretion. There is substantial accumulation of this
metabolite in renal failure.
Renal clearance of zidovudine greatly exceeds creatinine
clearance, indicating that significant tubular secretion
takes place. | [storage]
Store at -20°C | [References]
1) Yarchoan?et al. (1989),?Clinical Pharmacology of 3-Azido-2’,3’-Dideoxythymidine (Zidovudine) and Related Dideoxynucleosides; N. Engl. J. Med.?321?726
2) D’Andrea?et al.?(2008),?AZT: an old drug with new perspectives; Curr. Clin. Pharmacol.?3?20
3) Yu?et al. (2015),?Small molecules enhance CRISPR genome editing in pluripotent stem cells; Cell Stem Cell.?16?142 |
|
|