| Identification | Back Directory | [Name]
Propargyl-3-sulfopropyl ether sodium salt | [CAS]
30290-53-0 | [Synonyms]
POPS
popsliquid
30290-53-0
Propargyl-3-Sulfopropyl
Propargyl (3-sulfopropyl) ether
Propargyl-3-sulfopropyl ether sodium
PROPARGYL (3-SULFOPROPYL) ETHER, SODIUM SALT
SODIUM 3-(PROP-2-YNYLOXY)PROPANE-1-SULFONATE
Sodium 3-(prop-2-yn-1-yloxy)propane-1-sulfonate
POPS(Propargyl-3-sulfopropyl ether, sodium salt)
3-(2-Propynyloxy)-1-propanesulfonic acid, sodium salt
sodium 3-(1-sulfohex-5-yn-3-yloxy)-5-hexyne-1-sulfonate
sodium,3-(1-sulfohex-5-yn-3-yloxy)hex-5-yne-1-sulfonate
Propargyl (3-sulfopropyl) ether sodiuM salt solution,~45% in water | [EINECS(EC#)]
608-454-7 | [Molecular Formula]
C12H17NaO7S2 | [MDL Number]
MFCD00798857 | [MOL File]
30290-53-0.mol | [Molecular Weight]
360.38 |
| Chemical Properties | Back Directory | [density ]
1.21 at 20℃ | [vapor pressure ]
0-2992.94Pa at 25℃ | [form ]
Liquid | [InChI]
InChI=1S/C6H10O4S.Na.H/c1-2-4-10-5-3-6-11(7,8)9;;/h1H,3-6H2,(H,7,8,9);; | [InChIKey]
QZGFGSLFVLDKEY-UHFFFAOYSA-N | [SMILES]
C(S(O)(=O)=O)CCOCC#C.[NaH] | [LogP]
-4.1 | [Surface tension]
72.53-72.79mN/m at 1g/L and 19.9℃ |
| Hazard Information | Back Directory | [Description]
Propargyl-3-sulfopropyl ether sodium salt is a sulfonate used as a co-brightener with glycerol in the electroplating industry. It is also used as an alkaline industrial chemical that can be used to remove tellurium from glass and ceramics. Propargyl-3-sulfopropyl ether sodium salt is prepared by reacting propargyl alcohol with sodium sulfite and ethylene diamine. This reaction produces propargyl sulfonic acid, which reacts with ethylene oxide to form the desired product.
| [Uses]
Propargyl-3-sulfopropyl ether sodium salt is a sulfonate used as a co-brightener with glycerol in the electroplating industry. It is also used as an alkaline industrial chemical for removing tellurium from glass and ceramics. | [Synthesis]
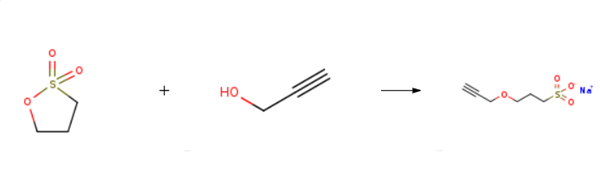
Propargyl-3-sulfopropyl ether sodium salt is prepared by the reaction of 1,3-propanesultone and propargyl alcohol. The specific synthesis steps are as follows:
To a chilled (5 °C) slurry of NaH (60% in mineral oil, 1.60 g, 40.9 mmol, 1.0 equiv.) in dry DMF (30 mL) was added dropwise a solution of propargyl alcohol (2.4 mL, 40.9 mmol, 1.0 equiv.) in dry DMF (30 mL) over a ten minute period. I ,3-Propanesultone (5.0 g, 40.9 minol, 1.0 equiv.) was dissolved in dry DMF (30mL), and the resulting solution added dropwise over a five minute period to the chilled sodium propargylate solution. After stirring for ten minutes in the ice bath, the flask was transferred to an oil bath and heated at 60°C for three hours. Most of the DMF was then removed by rotavap, and the remaining oil was triturated with 200 mL diethyl ether to yield a white solid, which was collected by vacuum filtration. The product was driedunder vacuum to provide Propargyl-3-sulfopropyl ether sodium salt as a white powder (7.4 g, 90%).
 |
|
|