| Identification | More | [Name]
Ethyl 1-benzylpiperidine-4-carboxylate | [CAS]
24228-40-8 | [Synonyms]
1-BENZYLPIPERIDINE-4-CARBOXYLIC ACID ETHYL ESTER
AKOS 225-01
BUTTPARK 33\04-91
ETHYL 1-BENZYL-4-PIPERIDINECARBOXYLATE
ETHYL 1-BENZYLPIPERIDINE-4-CARBOXYLATE
RARECHEM AH CK 0037
Benzylpiperidinecarboxylicacidethylester
N-Benzyl-4-cabethoxypiperidine
1-(Phenylmethyl)-4-piperidinecarboxylic Acid Ethyl Ester
1-(Phenylmethyl)piperidine-4-carboxylic Acid Ethyl Ester
Ethyl 1-Benzylisonipecotate
Ethyl 1-Benzylpiperidine-4-carboxylat
N-Benzyl-4-carboethoxypiperidine
1-Benzyl-4-piperidinecarboxyli
N-Benzyl-4-piperidinecarboxylicacidethylester
Methyl(ethyl) 1-benzyl-Piperidine-4-carboxylate
1-BENZYL-PIPERIDINE-4-CARBOXYLIC ACID ETHYL ESTER >98% | [EINECS(EC#)]
246-094-9 | [Molecular Formula]
C15H21NO2 | [MDL Number]
MFCD00040748 | [Molecular Weight]
247.33 | [MOL File]
24228-40-8.mol |
| Questions And Answer | Back Directory | [Synthesis]
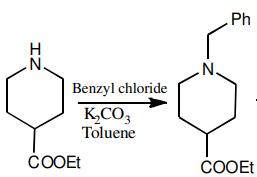
Ethyl isonipecotate (50 g, 0.31 mol) was dissolved in toluene (150 mL) in a round
bottom flask, charged with potassium carbonate (60 g, 0.43
mol) and stirred for 15 min. Benzyl chloride (42 g, 0.31 mol)
was charged and the reaction mass was refluxed for 4 h at
100 °C. Upon completion of the reaction as marked by TLC
(hexane:ethyl acetate; 2:1), the reaction mass was cooled to
room temperature and quenched with water (100 mL), stirred
and the organic phase was separated. The aqueous phase was
again extracted with toluene (100 mL). Combined organic
phase was washed twice with saturated brine solution (50 mL).
Remove toluene in vacuo to obtain Ethyl N-benzylpiperidine-4-carboxylate
( 6.97 g, 91 %) as a yellow liquid.
 |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
UN 2810 6.1 / PGIII | [Hazard Note ]
Irritant | [HS Code ]
29333990 |
|
|