| Identification | More | [Name]
3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate | [CAS]
2386-87-0 | [Synonyms]
3,4-EPOXYCYCLOHEXYLMETHYL 3',4'-EPOXYCYCLOHEXANECARBOXYLATE
3,4-EPOXYCYCLOHEXYLMETHYL 3,4-EPOXYCYCLOHEXANECARBOXYLATE
3,4-EPOXYCYCLOHEXYLMETHYL-3,4-EPOXYCYCLOHEXYLCARBOXYLATE
7-oxabicyclo[4.1.0]hept-3-ylmethyl 7-oxabicyclo[4.1.0]heptane-3-carboxylate
EMBEDDING MEDIUM ERL-4221D
3,4-epoxycyclohexanecarboxylicacid(3,4-epoxycyclohexylmethyl)ester
3,4-epoxycyclohexanemethyl3,4-epoxycyclohexanecarboxylate
3,4-epoxycyclohexylmethyl3,4-epoxycyclo-hexaneca
7-oxabicyclo(4.1.0)heptane-3-carboxylicacid,7-oxabicyclo(4.1.0)hept-3-ylmethy
7-Oxabicyclo[4,1,0]heptane-3-carboxylicacid,7-oxabicyclo[4,1,0]hept-3-ylmethylester
chissonox221monomer
erl-4221
ut632
3,4-EPOXYCYCLOHEXYLMETHYL
CYCLOALIPHATIC EPOXY RESIN
EPOXYCYCLOHEXYLMETHYL-EPOXYCYCLO-HEXANECARBOXYLATE
7-OXABICYCLOHEPTANE-3-CARBOXYLICACID,7-OXABICYCLOHEPT-3-YLMETHYLESTER
7-OXABICYCLOHEPTANE-3-CARBOXYLICACID
7-Oxabicyclo(4.1.0)hept-3-ylmethyl-7-oxabicyclo(4.1.0)heptan-3-carboxylat
7-OXABICYCLO[4.1.0]HEPTANE-3-CARBOXYLIC ACID | [EINECS(EC#)]
219-207-4 | [Molecular Formula]
C14H20O4 | [MDL Number]
MFCD00081118 | [Molecular Weight]
252.31 | [MOL File]
2386-87-0.mol |
| Chemical Properties | Back Directory | [Melting point ]
−37 °C(lit.)
| [Boiling point ]
355.49°C (rough estimate) | [density ]
1.17 g/mL at 25 °C(lit.)
| [vapor pressure ]
0.002Pa at 25℃ | [refractive index ]
n20/D 1.498(lit.)
| [Fp ]
245 °F
| [storage temp. ]
Sealed in dry,2-8°C | [solubility ]
Chloroform (Slightly), Dichloromethane (Slightly) | [form ]
Oil | [pka]
4.61±0.20(Predicted) | [color ]
Colourless | [Specific Gravity]
1.170 | [Water Solubility ]
13.85g/L at 20.2℃ | [InChIKey]
NHJIDZUQMHKGRE-UHFFFAOYSA-N | [LogP]
1.34 at 20℃ | [CAS DataBase Reference]
2386-87-0(CAS DataBase Reference) | [EPA Substance Registry System]
2386-87-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R43:May cause sensitization by skin contact. | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves . | [WGK Germany ]
1
| [RTECS ]
RN7750000
| [HS Code ]
29189900 | [Hazardous Substances Data]
2386-87-0(Hazardous Substances Data) | [Toxicity]
LD50 oral in rat: 4490mg/kg |
| Hazard Information | Back Directory | [Description]
3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate TTA21S is a pale yellow to colorless transparent liquid. Two package and UV cure formulas use TTA21 to create better crosslinks and enhance adhesion. Each molecule creates high cross-linking due to the dual epoxide functionality, which can react with hydroxyl, carboxyl, or amine groups of polymers in the system. End users take advantage of the UV stability and low viscosity compared to other epoxy resins.
| [Chemical Properties]
Pale yellow to colorless transparent liquid | [Uses]
This compound can be made by treatment of the corresponding
bis-unsaturated ester with peracetic acid. It is used as a
reactive diluent, for filament winding, as an acid scavenger,
or as a plasticizer. Vapor hazard is slight at ambient temperature
because of its moderately high boiling point. Skin
contact is to be avoided. | [General Description]
3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate (EEC) is a cycloaliphatic epoxy that can be synthesized by the reaction of 3′-cyclohexenylmethyl 3-cyclohexenecarboxylate with peracetic acid. Its aliphatic backbone and molecular structure provide a number of useful properties such as thermal stability, weatherability, and electrical conductivity. | [Flammability and Explosibility]
Nonflammable | [Synthesis]
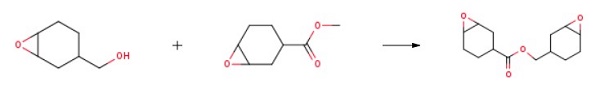
128.17kg of 3,4-epoxycyclohexyl-1-methanol, 312.36kg of 3,4-epoxycyclohexyl-1-methyl carboxylate, 9.61kg of tetrabutoxytitanium were added to the 500L reactor, and reacted at 100°C 7 hours (there is no apparent liquid steaming out at this time, sampling GC detection 3,4-epoxycyclohexyl-1-methanol content≤1.0wt%), stop the reaction; The reaction solution is at 500Pa vacuum, 95 vacuum distillation to remove excess Methyl 3,4-epoxycyclohexyl-1-carboxylate, the concentrated solution was distilled by molecular distillation, vacuum distillation at 2Pa, 144°C under reduced pressure to obtain 3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate.
|
|
|