| Identification | More | [Name]
Trifluoromethyl iodide | [CAS]
2314-97-8 | [Synonyms]
IODOTRIFLUOROMETHANE
PERFLUOROMETHYL IODIDE
TRIFLUOROMETHYL IODIDE
CF3I
CIF3
Freon13I1
freon13t1
iodotrifluoromethane(trifluoromethyliodide)
Methane,trifluoroiodo-
monoiodotrifluoromethane
R13I1
Trifluoriodmethan
Trifluoriodomethane
trifluoroiodo-methan
TRIFLUOROMETHYL IODIDE CYLINDER WITH
Trifluoromethyliodide,min.97%
Iodotrifluoromethane 99%
Iodotrifluoromethane99%
Trifluoroiodomethane, 99.9%
Trifluoroiodomethane, 97+% | [EINECS(EC#)]
219-014-5 | [Molecular Formula]
CF3I | [MDL Number]
MFCD00001060 | [Molecular Weight]
195.91 | [MOL File]
2314-97-8.mol |
| Chemical Properties | Back Directory | [Appearance]
colourless gas | [Melting point ]
<−78 °C(lit.)
| [Boiling point ]
−22.5 °C(lit.)
| [density ]
2.361 | [vapor pressure ]
540.5kPa at 25℃ | [refractive index ]
1.379 | [Fp ]
-22.5°C | [form ]
Gas | [Stability:]
Stable. Substances to be avoided include strong oxidizing agents. Avoid direct sunlight. Risk of explosion if heated under confinement. Flammable. | [Water Solubility ]
Slightly soluble in water. | [Sensitive ]
Light Sensitive | [BRN ]
1732740 | [LogP]
2.41 at 22.85℃ | [CAS DataBase Reference]
2314-97-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Methane, trifluoroiodo-(2314-97-8) | [EPA Substance Registry System]
2314-97-8(EPA Substance) |
| Questions And Answer | Back Directory | [Uses]
Trifluoroiodomethane is used as a gaseous fire suppression flooding agent for in-flight aircraft and electronic equipment fires. It is also an important raw material and intermediate used in organic synthesis, pharmaceuticals and agrochemicals. It is involved in the rhodium-catalyzed alfa-trifluoromethylation of alfa,beta-unsaturatedketones. It plays an important role as catalyst in the enantioselective alfa -trifluoromethylation of aldehydes through photoredox organocatalysis using a readily available iridium photocatalyst.
| [Reactions]
Trifluoromethyl iodide reacts with [AuMeL] to give [AuMe2(CF3)L] and [AuIL](L = PMe3 or PMe2Ph), or [Au(CF3)L] and Mel (L = PPh3), or a mixture of these products (L = PMePh2). In some cases reaction of [AuMe(PMe3)] with CF3I gives [AuMe(CF3)I(PMe3)]. Evidence is presented that the reactions proceed, at least in part, by a free-radical chain mechanism.
|
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R68:Possible risk of irreversible effects. | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves . | [RIDADR ]
UN 1956 2.2
| [WGK Germany ]
1
| [RTECS ]
PB6975000
| [F ]
27 | [Hazard Note ]
Irritant | [TSCA ]
T | [HazardClass ]
2.2 | [HS Code ]
2903780020 |
| Hazard Information | Back Directory | [Chemical Properties]
colourless gas | [Preparation]
Trifluoromethyl iodide's synthesis method:A glass flask provided with a gas outlet is filled with 80 g. (0.153 mole) of CI4 and 30 g. (0.135 mole) of IF5. The gas outlet is connected via short rubber tubes to several gas traps cooled with liquid nitrogen. Agitation of the vessel produces vigorous evolution of gas. When the reaction subsides, the system is heated for 30 min at 90-100°C. The condensate in the gas traps is then washed with 5% NaOH and fractionated. The yield is 90%.
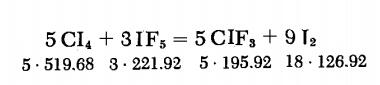
| [Synthesis Reference(s)]
Journal of the American Chemical Society, 107, p. 5014, 1985 DOI: 10.1021/ja00303a042 | [Flammability and Explosibility]
Notclassified |
|
|