| Identification | More | [Name]
2,3,4-Trihydroxybenzaldehyde | [CAS]
2144-08-3 | [Synonyms]
2,3,4-TRIHYDROXYBENZALDEHYDE
PYROGALLOL-4-CARBOXALDEHYDE
PYROGALLOLALDEHYDE
2,3,4-Trihydroxybenazldehyde
2,3,4-THB
Pyrogallolcarboxaldehyde
2,3,4-TRIHYDROXYBENZALDEHYDE 98% | [EINECS(EC#)]
218-404-2 | [Molecular Formula]
C7H6O4 | [MDL Number]
MFCD00003325 | [Molecular Weight]
154.12 | [MOL File]
2144-08-3.mol |
| Chemical Properties | Back Directory | [Appearance]
light brown to beige-brown powder | [Melting point ]
159-161 °C (lit.) | [Boiling point ]
237.46°C (rough estimate) | [density ]
1.3725 (rough estimate) | [refractive index ]
1.6400 (estimate) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
DMSO, Methanol | [form ]
Powder | [pka]
7.41±0.23(Predicted) | [color ]
Light brown to beige-brown | [Water Solubility ]
1.541g/L at 25℃ | [Sensitive ]
Air Sensitive | [BRN ]
2328658 | [LogP]
1.25 | [CAS DataBase Reference]
2144-08-3(CAS DataBase Reference) | [EPA Substance Registry System]
2144-08-3(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection .
S36:Wear suitable protective clothing . | [WGK Germany ]
2
| [RTECS ]
CU8439800
| [TSCA ]
Yes | [HS Code ]
29124990 |
| Hazard Information | Back Directory | [Description]
2,3, 4-trihydroxybenzaldehyde is an essential intermediate of the antiparkinson drug benserazide, which is a peripheral dopa decarboxylase inhibitor, usually benserazide: the L-dopa is prepared into a compound preparation at a ratio of 1:4, and has a remarkable effect of treating the Parkinson's disease.
| [Chemical Properties]
light brown to beige-brown powder | [Uses]
2,3,4-Trihydroxybenzaldehyde has been used in the preparation of 1,5-dimethyl-2-phenyl-4-[(1E)-(2,3,4-trihydroxybenzylidene)amino]-1H-pyrazol-3(2H)-one. | [Uses]
2,3,4-Trihydroxybenzaldehyde is a phenolic benzaldehyde with antibacterial activity. | [General Description]
Antimicrobial activity of carbohydrazone derived from 2,3,4-trihydroxybenzaldehyde against bacteria and fungi has been investigated. 2,3,4-trihydroxybenzaldehyde forms Schiff bases via [1+1] condensation with anthranilic acid. | [Synthesis]
The method for synthesizing 2,3, 4-trihydroxybenzaldehyde is shown as follows.
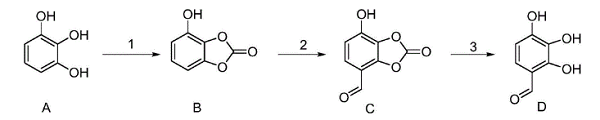 This method takes pyrogallol as an initial raw material. It comprises three steps of phenolic hydroxyl protection, formylation, and deprotection: protecting two adjacent phenolic hydroxyl groups in the pyrogallol to obtain 4-hydroxybenzo[d][1,3]dioxol-2-one, then performing formylation to obtain 7-hydroxy-2-oxobenzo[d][1,3]dioxole-4-carbaldehyde, and finally removing a protecting group to get a target 2,3,4-trihydroxybenzaldehyde. This method takes pyrogallol as an initial raw material. It comprises three steps of phenolic hydroxyl protection, formylation, and deprotection: protecting two adjacent phenolic hydroxyl groups in the pyrogallol to obtain 4-hydroxybenzo[d][1,3]dioxol-2-one, then performing formylation to obtain 7-hydroxy-2-oxobenzo[d][1,3]dioxole-4-carbaldehyde, and finally removing a protecting group to get a target 2,3,4-trihydroxybenzaldehyde.
|
| Spectrum Detail | Back Directory | [Spectrum Detail]
2,3,4-Trihydroxybenzaldehyde(2144-08-3)MS
2,3,4-Trihydroxybenzaldehyde(2144-08-3)1HNMR
2,3,4-Trihydroxybenzaldehyde(2144-08-3)13CNMR
2,3,4-Trihydroxybenzaldehyde(2144-08-3)IR1
2,3,4-Trihydroxybenzaldehyde(2144-08-3)IR2
|
|
|