| Identification | More | [Name]
N-BENZYLPHTHALIMIDE | [CAS]
2142-01-0 | [Synonyms]
BENZYLPHTHALIMIDE
N-BENZYLPHTHALIMIDE
SALOR-INT L253022-1EA
N-BENZYLPHTALIMIDE
2-Benzyl-1,3-isoindolinedione
2-Benzylisoindoline-1,3-dione | [EINECS(EC#)]
218-395-5 | [Molecular Formula]
C15H11NO2 | [MDL Number]
MFCD00023077 | [Molecular Weight]
237.25 | [MOL File]
2142-01-0.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystalline powder | [Melting point ]
114-116 °C(lit.) | [Boiling point ]
379.79°C (rough estimate) | [density ]
1.3430 | [refractive index ]
1.5500 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
powder to crystal | [pka]
-2.12±0.20(Predicted) | [color ]
White to Light yellow | [CAS DataBase Reference]
2142-01-0(CAS DataBase Reference) |
| Questions And Answer(Q&A) | Back Directory | [Preparation]
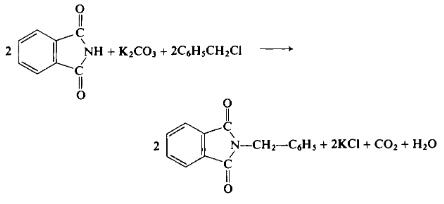
In a flask equipped with a reflux condenser, a mixture of 300 gm (2.04 mole) of phthalimide, 140 gm (1.09 mole) of potassium carbonate, and 300 gm (2.37 mole) of benzyl chloride is refluxed for 3 hr. The excess benzyl chloride is removed by steam distillation and the benzyl phthalimide which crystallizes out is filtered by suction. The product is washed with water, with 400 ml of 60% ethanol, and then dried to afford 360-375 gm (74-77%), m.p. 116°C (recrystallized from acetic acid).
|
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [HS Code ]
29251900 |
| Hazard Information | Back Directory | [Chemical Properties]
white crystalline powder | [Uses]
N-Benzylphthalimide may be used in the following syntheses:
- 2-benzyl-1,1,3,3-tetraphenylisoindoline
- tailor-made highly fluorous porphyrin derivatives
- N-benzylisoindole
| [Definition]
ChEBI: N-Benzylphthalimide is a member of isoindoles. | [Synthesis Reference(s)]
Organic Syntheses, Coll. Vol. 2, p. 83, 1943
The Journal of Organic Chemistry, 47, p. 2785, 1982 DOI: 10.1021/jo00135a021
Tetrahedron Letters, 11, p. 2691, 1970 | [General Description]
N-Benzylphthalimide (NBPT), also known as 2-benzylisoindoline-1,3-dione, is an N-substituted phthalimide. It has been prepared by reacting phthalic anhydride with benzyl amine in glacial acetic acid. Vibrational spectra of NBPT has been recorded and assigned. NBPT is a roof-shaped molecule with a planar cyclic imide and a phenyl ring connected by a methylene group. Crystal structure of N-benzylphthalimide has parallel layers of phthalimides stack along the a axis. |
|
|