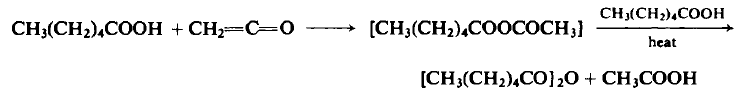| Identification | More | [Name]
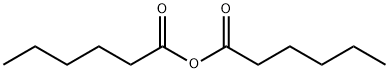
HEXANOIC ANHYDRIDE | [CAS]
2051-49-2 | [Synonyms]
CAPROIC ANHYDRIDE
HEXANOIC ANHYDRIDE
N-BUTYLACETIC ANHYDRIDE
N-CAPROIC ANHYDRIDE
N-CAPRONIC ANHYDRIDE
N-HEXANOIC ANHYDRIDE
N-HEXOIC ANHYDRIDE
PENTYLFORMIC ANHYDRIDE
Caproic acid anhydride
Capronic acid anhydride
Capronic anhydride
hexanoicacid,anhydride
Hexanoicacidanhydride
Hexanoyl anhydride
n-Capriocanhydride
n-Hexanoic acid anhydride
N-CAPROICANHYDRIDE=HEXANOICANHYDRIDE
Bis(hexanoic)anhydride
Biscaproic anhydride
Bishexanoic anhydride | [EINECS(EC#)]
218-121-4 | [Molecular Formula]
C12H22O3 | [MDL Number]
MFCD00009509 | [Molecular Weight]
214.3 | [MOL File]
2051-49-2.mol |
| Chemical Properties | Back Directory | [Appearance]
clear colorless to light yellow liquid | [Melting point ]
-40 °C | [Boiling point ]
246-248 °C (lit.) | [density ]
0.928 g/mL at 20 °C(lit.)
| [vapor pressure ]
2.9Pa at 25℃ | [refractive index ]
n20/D 1.428(lit.)
| [Fp ]
>230 °F
| [storage temp. ]
Store below +30°C. | [solubility ]
ethanol: soluble1g/10 mL, clear, colorless | [form ]
Liquid | [color ]
Clear colorless to light yellow | [explosive limit]
0.7%(V) | [Water Solubility ]
Hydrolyzes in water. | [Sensitive ]
Moisture Sensitive | [BRN ]
1776561 | [LogP]
4.45 at 25℃ | [CAS DataBase Reference]
2051-49-2(CAS DataBase Reference) | [EPA Substance Registry System]
Hexanoic acid, anhydride (2051-49-2) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3265 8/PG 2
| [WGK Germany ]
3
| [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
29159000 |
| Questions And Answer(Q&A) | Back Directory | [preparation]
To an ice-cooled flask containing 116 gm (1.0 mole) of n-caproic acid is added 21.0-23.10 gm (0.5-0.55 mole) of ketene at a rate of 0.45 mole/hr. The reaction mixture is fractionally distilled at atmospheric pressure to afford a forecut of acetone, acetic acid, and acetic anhydride. The oil bath is raised to 220°C over a 1-hr period, kept there for 3 hr to ensure complete removal of acetic acid, and then cooled. The distillation is continued under reduced pressure to afford 86-95 gm (80-87%), b.p. 109- 112°C (3 mm Hg) and b.p. 118-121°C (6 mm Hg).
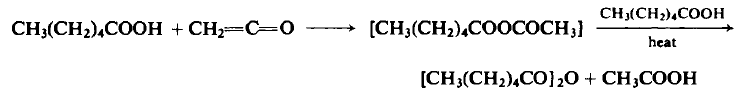
|
| Hazard Information | Back Directory | [Chemical Properties]
clear colorless to light yellow liquid | [Uses]
Hexanoic anhydride has been used in:
- green synthesis of esters of acyclovir (acyclovir prodrugs)
- preparation of hexanoyl-modified chitosan nanoparticles and chitosan-based polymeric surfactants via N-acylation of chitosans
| [Uses]
Hexanoic anhydride was used in:
- green synthesis of esters of acyclovir (acyclovir prodrugs)
- preparation of hexanoyl-modified chitosan nanoparticles
- preparation of chitosan-based polymeric surfactants via N-acylation of chitosans
| [Uses]
Hexanoic Anhydride, is used as a reactant in the total synthesis of acremomannolipin A via steroselective β-mannosylation of 4,6,-O-benzylidene-protected mannosyl sulfoxide with a D-mannitol derivative. | [Flammability and Explosibility]
Notclassified |
|
|