| Identification | More | [Name]
4'-BROMO-3'-NITROACETOPHENONE | [CAS]
18640-58-9 | [Synonyms]
1-(4-BROMO-3-NITROPHENYL)ETHAN-1-ONE
1-(4-BROMO-3-NITROPHENYL)ETHANONE
4'-BROMO-3'-NITROACETOPHENONE
4-BROMO-3-NITROACETOPHENONE
3-Nitro-4-bromoacetophenone
Acetophenone, 4'-bromo-3'-nitro-
Ethanone, 1-(4-bromo-3-nitrophenyl)-
4'-Bromo-3'-nitroacetophenone, 99+%
4'-bromo-3'-nitroacetophenone,1-(4-bromo-3-nitrophenyl)ethanone | [EINECS(EC#)]
242-469-6 | [Molecular Formula]
C8H6BrNO3 | [MDL Number]
MFCD00016985 | [Molecular Weight]
244.04 | [MOL File]
18640-58-9.mol |
| Chemical Properties | Back Directory | [Appearance]
white to slightly yellow amorphous powder | [Melting point ]
117-121 °C(lit.)
| [Boiling point ]
275.4℃ | [density ]
1.637 | [refractive index ]
1.6090 (estimate) | [Fp ]
120.4℃ | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
Amorphous Powder | [color ]
White to slightly yellow | [BRN ]
2050098 | [InChI]
InChI=1S/C8H6BrNO3/c1-5(11)6-2-3-7(9)8(4-6)10(12)13/h2-4H,1H3 | [InChIKey]
YFVOFFKNHQTQQE-UHFFFAOYSA-N | [SMILES]
C(=O)(C1=CC=C(Br)C([N+]([O-])=O)=C1)C | [CAS DataBase Reference]
18640-58-9(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [HS Code ]
29147000 |
| Hazard Information | Back Directory | [Chemical Properties]
white to slightly yellow amorphous powder | [General Description]
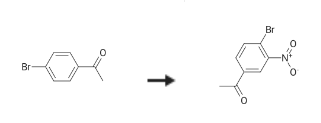
4′-Bromo-3′-nitroacetophenone is an electron deficient acetophenone derivative. | [Synthesis]
4'-Bromo-3'-nitroacetophenone is synthesised using 4'-Bromoacetophenone as a raw material by chemical reaction. The specific synthesis steps are as follows:
To fuming nitric acid (200 mL), l-(4- bromophenyl)-l-propanone (1-1) (40 g, 0.20 mol) was added while keeping the inside temperature of mixture at 5 to 10 0C. The reaction solution was stirred at this temperature for 30 minutes and then poured into ice. The precipitate was collected by filtration, washed with distilled water (25 mL x 2) and re-crystallized from methanol to give 1-2 (18 g, 37percent yield). 1H NMR (500 MHz, CDCl3) δ 8.38 (IH, d, J= 2.0 Hz), 7.99 (IH, dd, J= 8.2 Hz, 2.0 Hz), 7.86 (IH, d, J= 8.2Hz), 3.01 (2H, q, J= 7.1Hz), 1.25 (3H, t, J= 7.1Hz) ppm; LC-MS (ESI): m/z 244.0 (M+H)+.
 |
|
|