| Identification | More | [Name]
3-Bromo-5-fluorobenzonitrile | [CAS]
179898-34-1 | [Synonyms]
3-BROMO-5-FLUOROBENZONITRILE
3-Bromo-5-fluorobenzonitrile 98%
3-Bromo-5-fluorobenzonitrile98% | [Molecular Formula]
C7H3BrFN | [MDL Number]
MFCD04038227 | [Molecular Weight]
200.01 | [MOL File]
179898-34-1.mol |
| Safety Data | Back Directory | [Hazard Codes ]
T,Xi | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
3439 | [Hazard Note ]
Toxic | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
2926907090 |
| Hazard Information | Back Directory | [Chemical Properties]
off-white crystalline | [Uses]
3-Bromo-5-fluorobenzonitrile is a compound of benzonitrile containing bromine and fluorine atoms, which is mainly used as an intermediate component in organic synthesis. | [Synthesis]
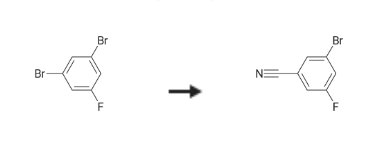
3-Bromo-5-fluorobenzonitrile is synthesised using 1,3-dibromo-5-fluorobenzene as a raw material by chemical reaction. The specific synthesis steps are as follows:
A 250-mL round-bottom flask equipped with a magnetic stir bar was charged with 1,3-dibromo-5-fluorobenzene (7.70 g, 30.3 mmol), DMF (45 mL), pyridine (4.9 mL), and copper (I) cyanide (2.72 g, 30.3 mmol) under nitrogen.
A reflux condenser was attached to the flask.
The green, cloudy mixture was stirred at reflux for 3 h.
Once lower Rf impurities were observed, the reaction was allowed to cool to room temperature.
The reaction was quenched with 30 mL of ether, and a precipitate formed in the dark solution.
The precipitate was gravity-filtered though Celite.
The filtrate was rinsed three times with ether (100 mL/50 g bromide).
The isolated solution was added to a separatory funnel.
The organic layer was washed with a 2:1 mixture of water and concentrated ammonium hydroxide (30 mL), followed by saturated ammonium chloride solution (2*30 mL) and saturated sodium bicarbonate (30 mL).
The aqueous layers were extracted with ether (3*40 mL).
The organic layers were combined and dried over anhydrous sodium sulfate.
The product was purified by flash column chomatography to yield 3-bromo-5-fluorobenzonitrile (2.10 g, 35percent).
1H NMR (400 MHz, CDCl3) δ 7.62 (s, 1H), 7.54-7.50 (m, 1H), 7.35-7.32 (m, 1H).
 |
|
|