| Identification | More | [Name]
cis-1,2-Cyclohexanediol | [CAS]
1792-81-0 | [Synonyms]
CIS-1,2-CYCLOHEXANEDIOL
CIS-1,2-DIHYDROXYCYCLOHEXANE
CIS-HEXAHYDROBRENZCATECHIN
CIS-HEXAHYDROCATECHOL
TIMTEC-BB SBB007678
1,2-Cyclohexanediol, cis-
Grandidentol
cis-hexahydrobrenzkatechin
(1R,2S)-cyclohexane-1,2-diol
1,2-cis-Dihydroxycyclohexane
1,2-Cyclohexanediol, (1R,2S)-
cis-2-Hydroxycyclohexanol
meso-cis-1,2-Cyclohexanediol
(1R,2S)-1,2-Cyclohexanediol
(1S)-1α,2α-Cyclohexanediol
(1α,2α)-1,2-Cyclohexanediol | [Molecular Formula]
C6H12O2 | [MDL Number]
MFCD00064944 | [Molecular Weight]
116.16 | [MOL File]
1792-81-0.mol |
| Questions And Answer | Back Directory | [Synthesis]
To a mixture of N-methylmorpholine-N-oxide.2H2O (18.2 g, 155 mmol), water (50 mL), acetone (20 mL), and osmium tetroxide (80 mg) in t-butanol (8 mL) was added distilled cyclohexene (10.1 mL, 100 mmol). The reaction was slightly exothermic initially and was maintained at room temperature with a water bath. The reaction was complete after stirring overnight at room temperature under nitrogen. A slurry of 1 g of sodium hydrosulfifite, 12 g of magnesium silicate (magnesol), and 80 ml of water was added, and the magnesol was fifiltered. The fifiltrate was neutralized to pH 7 with 1 N H2SO4, the acetone was evaporated under vacuum, and the pH was further adjusted to pH 2. The solution was saturated with NaCl and extracted with EtOAc. The aqueous phase was concentrated by azeotroping with n-butanol and further extracted with ethyl acetate. The combined ethyl acetate layers were dried and evaporated, yielding 11.2 g (96.6%) of crystalline solid. Recrystallization from ether provided 10.6 g (91%) of cis-1,2-cyclohexanediol, mp 95–97°C.
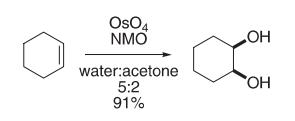
Reference: Van Rheenen, V.; Kelly, R. C.; Cha, D. Y. Tetrahedron Lett. 1976, 17, 1973?1976. |
| Safety Data | Back Directory | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [F ]
3-10 | [HS Code ]
29061990 |
| Hazard Information | Back Directory | [Chemical Properties]
White to light beige crystalline flakes or powder | [Uses]
cis-1,2-Cyclohexanediol is a reagent used in the synthesis of boronic esters of corannulene which are used to prepare icosahedral supramolecules. | [Definition]
ChEBI: A cyclohexane-1,2-diol with cis-configuration. | [General Description]
Core-shell-like silica nickel species nanoparticle catalyzed dehydrogenation of 1,2-cyclohexanediol to catechol is reported. Crystal structure of a Cr(V) complex with cis-1,2-cyclohexanediol is reported. Enzymatic oxidation of cis-1,2-cyclohexanediol by Gluconobacter oxydans (ATCC 621) is reported. |
|
|