| Identification | Back Directory | [Name]
(R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-COBALT(II) | [CAS]
176763-62-5 | [Synonyms]
(R�����,R)-(-)-N��,N&rsquo
CyclohexanediaminoNNbisditbutylsalicylidene
(1R,2R)-(-)-1,2-Cyclohexanediamino-N,N'-bis(3,5-
(1r,2r)-(-)-1,2-cyclohexanediamino-n,n'-bis(3,5- di-t-butylsalicylidene)cobalt
(1R,2R)-(-)-N,N'-BIS(3,5-DI-T-BUTYLSALICYDENE)-1,2-CYCLOHEXANEDIAMINOCOBALT(II)
(1R,2R)-(-)-N,N-Bis(3,5-di-t-butylsalicylidene)-1,2-cyclohexanediaminocobalt(II)
(R,R)-(-)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminocobalt(Ⅱ)
(1R,2R)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-
cyclohexanediaminocobalt(II)
(1R,2R)-(-)-1,2-CYCLOHEXANEDIAMINO-N N'-BIS(3,5-DI-T-BUTYLSALICYLIDENE)COBALT(II)
(R,R)-(-)-N,N'-BIS(3,5-DI-T-BUTYLSALICYL ID -ENE)-1,2-CYCLOHEXANEDIAMINOCOBALT(II
(1R,2R)-(-)-1,2-Cyclohexanediamino-N,N'-bis(3,5-di-t-butylsalicylidene) cobalt(Ⅱ)
(R,R)-(-)-N,N'-Bis(3,5-di-t-butylsalicylidene)-1,2-cyclohexanediaminocobalt(ii),98%
(R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-COBALT(II)
(1R,2R)-(-)-1,2-Cyclohexanediamino-N,N'-bis(3,5-di-tert-butylsalicylidene)cobalt(II)
(R,R)-(-)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminocobalt(II),98%
(R,R)-(-)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminocob alt(II)
(1R,2R)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-
cyclohexanediaminocobalt(II),99%e.e.
Cobalt,[[2,2'-[(1R,2R)-1,2-cyclohexanediylbis[(nitrilo-kN)Methylidyne]]bis[4,6-bis(1,1-diMethylethyl)phenolato-kO]](2-)]-, (SP-4-2)- | [Molecular Formula]
C36H52CoN2O2 | [MDL Number]
MFCD01631277 | [MOL File]
176763-62-5.mol | [Molecular Weight]
603.74 |
| Chemical Properties | Back Directory | [Appearance]
Orange to red-brown powder | [Melting point ]
>350 °C(lit.)
| [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [form ]
Powder | [color ]
Orange to red to brown | [InChIKey]
ZFWPDJKMASHRPT-DKZUAMKGNA-L | [SMILES]
C12O[Co]OC3=C(C=N[C@@H]4CCCC[C@H]4N=CC=1C=C(C(C)(C)C)C=C2C(C)(C)C)C=C(C(C)(C)C)C=C3C(C)(C)C |t:6,15,&1:8,13,r| |
| Hazard Information | Back Directory | [Chemical Properties]
Orange to red-brown powder | [Uses]
Catalyst for the hydrolytic kinetic resolution of terminal epoxides and the enantioselective ring opening of meso epoxides. |
| Questions And Answer | Back Directory | [Reaction]
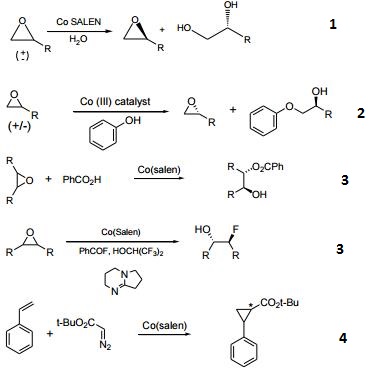
- Catalyst used in the kinetic resolution of racemic, terminal epoxides yielding a chiral diol and the unreacted enantiomer of the epoxide.
- Precursor to a Co(III) catalyst for the kinetic resolution of terminal epoxides with alcohols.
- Desymmetrization of meso-epoxides with carboxylic acids and fluoride.
- Catalyst for asymmetric cyclopropanation of styrene.
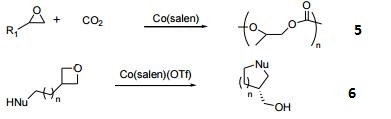
- Catalyst for copolymerization of CO2 and epoxides.
- Enantioselective intramolecular openings of oxetanes.
|
|
|