| Identification | Back Directory | [Name]
5-(2R)-2-Oxiranyl-8-benzyloxy-2(1H)-quinolinone | [CAS]
173140-90-4 | [Synonyms]
BNKY004-IM01
8-benzoxy-5-(R)-oxiranyl-1H-quinolin-2-one
5-(2R)-2-Oxiranyl-8-benzyloxy-2(1H)-quinolinone
(R)-5-(2-oxiranyl)-8-benzyloxy-2(1H)-quinolinone
2 5-(2R)-2-Oxiranyl-8-benzyloxy-2(1H)-quinolinone
(R)-8-(Benzyloxy)-5-(oxiran-2-yl)quinolin-2(1H)-one
8-(Benzyloxy)-5-[(2R)-oxiran-2-yl]quinolin-2(1H)-one
2(1H)-Quinolinone, 5-(2R)-oxiranyl-8-(phenylmethoxy)-
5-[(2R)-oxiran-2-yl]-8-phenylmethoxy-1H-quinolin-2-one
2(1H)-Quinolinone, 5-(2R)-2-oxiranyl-8-(phenylmethoxy)-
Indacaterol interMediate :2
5-(2R)-2-Oxiranyl-8-benzyloxy-2(1H)-quinolinone | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C18H15NO3 | [MDL Number]
MFCD17018704 | [MOL File]
173140-90-4.mol | [Molecular Weight]
293.32 |
| Chemical Properties | Back Directory | [Melting point ]
155 - 157°C | [Boiling point ]
556.0±50.0 °C(Predicted) | [density ]
1.298 | [storage temp. ]
Hygroscopic, -20°C Freezer, Under inert atmosphere | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
10.92±0.70(Predicted) | [color ]
White to Pale Yellow | [Stability:]
Hygroscopic |
| Hazard Information | Back Directory | [Uses]
5-(2R)-2-Oxiranyl-8-benzyloxy-2(1H)-quinolinone is used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. | [Synthesis]
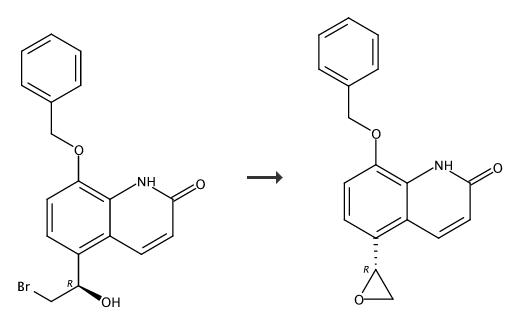
A 5 liter flask equipped with a mechanical stirrer, thermometer, and refluxing condenser was charged with 8-benzyloxy-5-[(R)-(2-bromo-1-hydroxyethyl)]-carbostyriI (70gms/0.187 moles), potassium carbonate (74 gmsl 0.536 moles), acetone (3.5 liters) and water (35 ml). The resulting slurry 'was heated to reflux and maintained for 2% hours. After completion of reaction, the hot mass was filtered on hylo bed to remove inorganics. The residue was slurried in dichloromethane (200 ml) and filtered on hyflo bed. The filtrates were combined together and concentrated under vacuum completely. The residue was dissolved. in dichloromethane (500ml) and filtered on hyflo bed to remove traces of insolubles and washed with dichloromethane(100 ml). The clear filtrate was distilled completely to obtain residue. The residue was charged with methanol (70 ml), stirred and heated to 50??C for 30 minutes. The slurry obtained was cooled to 25-30??C,chilled to 0- 5??C, stirred for 1 hour. The resulting solid was isolated by filtration, washed with methanol (30ml), followed by diisopropylether (100ml) and dried under vacuum at 60-65 ??C for 10-12 hours to yield 40-41 gms of 8-benzyloxy- 5-(R)-oxiranylcarbostyril. |
|
|