| Identification | Back Directory | [Name]
(+)-N,N'-(1S,2S)-1,2-DIAMINOCYCLOHEXANEDIYLBIS(2-PYRIDINECARBOXAMIDE) | [CAS]
172138-95-3 | [Synonyms]
(S,S)-DACH-pyridyl Trost ligand
(S,S)-DACH-pyridyl Trost ligand 95%
2,6-Bis[(4S)-benzyl-2-oxazolin-2-yl]-pyridine
N,N'-((1S,2S)-Cyclohexane-1,2-diyl)dipicolinaMide
(1S,2S)-1,2-Bis(2-pyridinecarboxamido)cyclohexane
N-((1S,2S)-2-(picolinamido)cyclohexyl)picolinamide
(1R,2R)-(-)-1,2-Bis[(2-pyridinylcarbonyl)amino]cyclohexane
1S,2S- N,N’-1,2-Diaminocyclohexanediylbis
(2-pyridinecarboxamide)
(+)-N,N'-(1S,2S)-1,2-DIAMINOCYCLOHEXANEDIYLBIS(2-PYRIDINECARBOXAMIDE)
(+)-N,N'-(1S,2S)-1,2-Diaminocyclohexanediylbis(2-pyridinecarboxamide),98%
(1S,2S)-N,N'-1,2-Diaminocyclohexanediylbis(2-
pyridinecarboxamide),99%e.e.
(+)-N,N'-(1S,2S)-1,2-Diaminocyclohexanediylbis(2-pyridinecarboxamide),min.98%
(+)-N,N''-(1S,2S)-1,2-DIAMINOCYCLOHEXANEDIYLBIS(2-PYRIDINECARBOXAMIDE), MIN. 98%
(1R,2R)-N,Nμ-1,2-Cyclohexanediylbis(2-pyridinecarboxamide), (1R,2R)-1,2-Bis(2-pyridinecarboxamido)cyclohexane
(+)-N,N'-(1S,2S)-1,2-Diaminocyclohexanediylbis(2-pyridinecarboxamide), min. 98% (S,S)-DACH-Pyridyl Trost Ligand
(1S,2S)-N,Nμ-1,2-Cyclohexanediylbis(2-pyridinecarboxamide), (+)-N,Nμ-(1S,2S)-1,2-Diaminocyclohexanediylbis(2-pyridinecarboxamide), (1S,2S)-(+)-1,2-Bis[(2-pyridinylcarbonyl)amino]cyclohexane, (1S,2S)-1,2-Bis(2-pyridinecarboxamido)cyclohexane | [Molecular Formula]
C18H20N4O2 | [MDL Number]
MFCD02684544 | [MOL File]
172138-95-3.mol | [Molecular Weight]
324.38 |
| Chemical Properties | Back Directory | [Melting point ]
170°C | [alpha ]
+95° (c 1, CH3OH) | [Boiling point ]
663.4±50.0 °C(Predicted) | [density ]
1.25±0.1 g/cm3(Predicted) | [storage temp. ]
2-8°C | [form ]
Powder | [pka]
12.51±0.40(Predicted) | [color ]
off-white | [CAS DataBase Reference]
172138-95-3 |
| Questions And Answer | Back Directory | [Reactions]
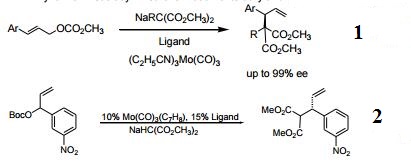
- Ligands for Mo catalyzed asymmetric allylic substitutions. Especially useful for the synthesis of tertiary and quaternary stereocenters. Exclusive license for this technology acquired by ChiroTech and is protected by pending Stanford University patents.
- Dynamic kinetic asymmetric formation of tertiary and quaternary stereogenic centers
|
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|