| Identification | More | [Name]
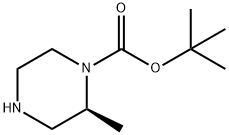
(S)-1-N-Boc-2-methylpiperazine | [CAS]
169447-70-5 | [Synonyms]
(R)-1-N-BOC-2-METHYL PIPERAZINE
(R)-2-METHYL-1-BOC-PIPERAZINE
(R)-2-METHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
(R)-TERT-BUTYL 2-METHYLPIPERAZINE-1-CARBOXYLATE
(S)-1-N-BOC-2-METHYL PIPERAZINE
(S)-2-METHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
(S)-TERT-BUTYL 2-METHYLPIPERAZINE-1-CARBOXYLATE
(S)-1-Boc-2-methyl-piperazine
Tert-Butyl2(S)-methylpiperazine-1-carboxylate
2-S-Methyl-piperazine-1-carboxylic acid tert-butyl ester
(S)-1-N-Boc-2-Methylpiperizine
tert-butyl 2(S)-methylpiperazine-1-carboxylate
(S)-N1-BOC-2-METHYLPIPERAZINE
(S)-1-Boc-2-methl-piperazine | [Molecular Formula]
C10H20N2O2 | [MDL Number]
MFCD01862120 | [Molecular Weight]
200.28 | [MOL File]
169447-70-5.mol |
| Chemical Properties | Back Directory | [Melting point ]
43.0 to 47.0 °C | [Boiling point ]
268.7±15.0 °C(Predicted) | [density ]
0.997±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
soluble in Methanol | [form ]
powder to crystal | [pka]
8.49±0.40(Predicted) | [color ]
White to Almost white | [InChI]
InChI=1S/C10H20N2O2/c1-8-7-11-5-6-12(8)9(13)14-10(2,3)4/h8,11H,5-7H2,1-4H3/t8-/m0/s1 | [InChIKey]
DATRVIMZZZVHMP-QMMMGPOBSA-N | [SMILES]
N1(C(OC(C)(C)C)=O)CCNC[C@@H]1C | [CAS DataBase Reference]
169447-70-5(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Uses]
(S)-1-N-Boc-2-methylpiperazine is used in the preparation of pyrazolo[3,4-b]pyridine dual pharmacophores as PDE4-muscarinic antagonists.
| [Synthesis]
A solution of (S)-2-methyl piperazine (2 g, 20 mmol) in THF (200 ml_) was mixed with nBuLi (25 ml, 1.6 M in hexanes, 40 mmol) at rt. The solution was stirred for 30 minutes before TBDMSCI (3.04 g, 20 mmol) was added. The mixture was stirred for 1 hour and then (Boc)2O (5.2 g, 24 mmol) was added to the solution. After 1 hour, the solution was diluted with H2O (50 ml). The organic layer was separated, washed with brine (50 mL), dried over Na2SO4, and concentrated EPO under vacuum. Finally, (S)-1-N-Boc-2-methylpiperazine was obtained after purification.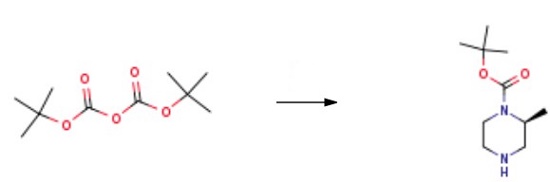 |
|
|